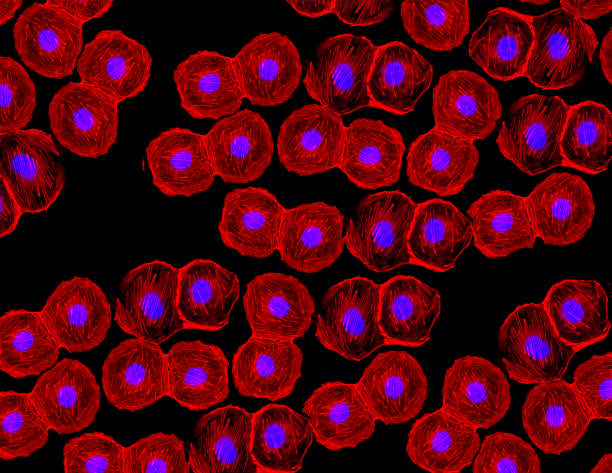
Imagine a cell so powerful that it can become almost any other cell in your body. A cell that, when triggered, can transform into a heart cell, a nerve cell, a muscle fiber, or even bone. It sounds like something out of science fiction—like biological shapeshifters that hold the secrets of regeneration. Yet these very real and remarkable entities exist within each of us. They are called stem cells, and they are the foundational architects of life.
Stem cells are among the most fascinating and promising discoveries in modern biology. Their ability to morph into specialized cells makes them essential to growth, healing, and possibly even immortality. As science unlocks their secrets, stem cells are poised to revolutionize medicine—from regenerating damaged organs to curing diseases once thought untreatable.
But to fully appreciate the power and potential of stem cells, we must first understand where they come from, how they work, and what makes them so uniquely potent in the symphony of life.
The Origins of Stem Cells: A Biological Beginning
All multicellular life starts from a single cell—a fertilized egg. From this one cell, an entire organism emerges: bones, blood, brain, and skin, each with a specific function. This miracle of development is made possible by the earliest and most potent form of stem cells: embryonic stem cells.
Embryonic stem cells are pluripotent, meaning they can give rise to nearly every type of cell in the body. In the early stages of an embryo’s development, these cells are like blank slates—flexible, adaptable, and ready to specialize. They divide rapidly and provide the raw materials for building tissues and organs.
As development progresses, the cells begin to commit to particular paths. Some become precursors to blood cells, others to nerve cells or muscle tissue. But even in adulthood, a reservoir of stem cells remains scattered throughout the body. These are adult stem cells, also called somatic stem cells. While not as versatile as embryonic stem cells, they still play a vital role in repair and regeneration.
One of the most well-known adult stem cells is the hematopoietic stem cell, found in bone marrow. It gives rise to all the different types of blood cells: red cells that carry oxygen, white cells that fight infection, and platelets that help blood clot. Without these stem cells, our blood system would fail.
The Different Faces of Stem Cells
Not all stem cells are created equal. Their ability to differentiate—the process of becoming a specialized cell—varies. Scientists classify stem cells based on their potency, or their potential to develop into different types of cells.
At the top of this hierarchy are totipotent stem cells, which can become any cell in the body and even give rise to an entire organism. The zygote, formed by the fusion of sperm and egg, is a totipotent cell.
Just below totipotent are pluripotent stem cells, like those found in early embryos. These can develop into any body cell but not into an entire organism, because they lack the extra-embryonic tissues like the placenta.
Then there are multipotent stem cells, which are more limited. They can develop into a group of related cell types. Hematopoietic stem cells, for example, are multipotent because they can become any type of blood cell but not a nerve or skin cell.
Unipotent stem cells are the most restricted. They can only become one type of cell—but they can self-renew, which sets them apart from ordinary cells.
Finally, there are induced pluripotent stem cells (iPSCs)—a groundbreaking discovery that changed the face of regenerative medicine. These are adult cells that scientists have reprogrammed back into a pluripotent state, giving them the versatility of embryonic stem cells without the ethical controversies.
The Science of Self-Renewal
One of the defining features of stem cells is their ability to self-renew. This means they can divide and produce more stem cells, maintaining a pool of undifferentiated cells even as some specialize.
This is no ordinary cell division. Most cells in the body divide a limited number of times before aging and dying. Stem cells, however, can keep dividing for long periods—some indefinitely. This ability is critical during development, where rapid growth is required, and in adulthood, where maintenance and repair are ongoing.
Self-renewal is tightly regulated by genes and molecular signals. If the controls fail, it can lead to uncontrolled growth—a hallmark of cancer. In fact, some cancers are thought to originate from “cancer stem cells,” which retain the self-renewing properties of normal stem cells but have lost the ability to differentiate properly.
Thus, stem cells walk a biological tightrope: too little division and the body cannot heal; too much, and tumors may form.
Stem Cells and Regeneration: Nature’s Repair Kit
What happens when you get a paper cut? Your body activates a complex cascade of events: platelets form a clot, white blood cells prevent infection, and skin cells regenerate to close the wound. Behind the scenes, stem cells are hard at work.
In many tissues, adult stem cells lie dormant until they are called upon to repair damage. In muscles, for instance, satellite cells serve as resident stem cells. When muscle fibers are injured, these cells activate, proliferate, and fuse to repair or replace the damaged tissue.
In the skin, epidermal stem cells regenerate the outer layer continuously. In the intestines, intestinal stem cells regenerate the lining every few days. Even the liver, known for its regenerative prowess, contains progenitor cells that can become liver cells when needed.
This regenerative capacity is not equal across the body. Some tissues, like the heart and brain, have limited stem cell activity. That’s why heart attacks and neurodegenerative diseases are so devastating. But stem cell therapies may soon change that.
Stem Cells in Medicine: A New Era of Healing
Stem cells offer tantalizing possibilities in medicine. The idea is simple yet profound: use stem cells to replace or regenerate damaged tissues. This field, known as regenerative medicine, is one of the fastest-growing frontiers in science.
Already, bone marrow transplants—which transfer hematopoietic stem cells—have been used for decades to treat leukemia and other blood disorders. These transplants reboot the patient’s blood and immune systems, often curing diseases that would otherwise be fatal.
Now, researchers are looking beyond the blood. Stem cell therapies are being explored for Parkinson’s disease, spinal cord injury, heart disease, diabetes, macular degeneration, and more. In some trials, stem cells have been used to grow cartilage in arthritic joints, create insulin-producing cells for diabetics, or repair damaged heart tissue after a heart attack.
There’s even work underway to grow organoids—miniature versions of organs like the brain, kidney, or liver—from stem cells. These organoids can be used for research, drug testing, or potentially even transplantation in the future.
Ethical Debates and Scientific Controversies
Stem cells are as ethically charged as they are scientifically potent. Much of the controversy stems from embryonic stem cells, which are harvested from early-stage embryos. Critics argue that this process destroys potential human life, while proponents say the medical benefits justify their use—especially when the embryos come from IVF clinics and would otherwise be discarded.
In response to these concerns, scientists have developed induced pluripotent stem cells (iPSCs), which avoid the ethical quandaries. iPSCs are created by reprogramming adult cells—like skin or blood cells—into a pluripotent state. This innovation won the Nobel Prize in Physiology or Medicine in 2012 and opened new avenues for research and therapy.
Still, challenges remain. Not all iPSCs behave exactly like embryonic stem cells. Some may have genetic abnormalities or behave unpredictably. The field must balance innovation with caution, ensuring safety and efficacy before widespread use.
The Promise and Perils of Stem Cell Clinics
As the public’s fascination with stem cells grows, so too has a troubling trend: the rise of unregulated stem cell clinics. These businesses promise miraculous cures for everything from arthritis to autism, often without scientific evidence or FDA approval.
In some cases, patients have been harmed by unproven stem cell treatments. Injections into the eyes, spine, or joints have led to infections, blindness, or tumors. The problem lies in the lack of regulation and oversight. While stem cell science is real, not every product sold as a “stem cell therapy” is legitimate or safe.
Authorities like the FDA are cracking down on these clinics, but the allure of a “miracle cure” remains strong. Patients must be educated and cautious, seeking treatments only from reputable, scientifically-backed institutions.
Personalized Medicine and the Future of Stem Cells
Stem cells are also paving the way for personalized medicine—tailoring treatments to an individual’s genetic makeup. iPSCs can be derived from a patient’s own cells, minimizing the risk of immune rejection. These patient-specific stem cells can then be used to test drugs, model diseases, or even develop custom therapies.
For example, scientists can take skin cells from a person with a rare genetic disorder, reprogram them into iPSCs, and then differentiate them into the affected cell type. By studying these cells in the lab, researchers can uncover the molecular roots of the disease and screen potential drugs—all without touching the patient.
This approach is especially valuable in genetic disorders, cancer research, and neurological diseases, where access to affected tissues is otherwise difficult.
Stem Cells in Space: Research Beyond Earth
In a fascinating twist, stem cells are also being studied in space. Astronauts aboard the International Space Station are conducting experiments to see how microgravity affects stem cell growth and differentiation. Understanding these effects could be key to maintaining astronaut health during long missions and may offer insights into aging and regeneration on Earth.
Some researchers believe that microgravity may even enhance stem cell proliferation or improve the production of organoids. As humanity pushes toward Mars and beyond, stem cells could play a crucial role in medicine, food production, and even life support systems in space.
A New Understanding of Life Itself
At its core, the study of stem cells is a study of potential—the potential to grow, to heal, to change. These tiny, powerful cells embody life’s ability to adapt and renew. They offer hope not just for treating disease, but for understanding the very principles that govern biology.
As our tools improve and our knowledge deepens, we may learn to guide stem cells with precision, harnessing their abilities safely and ethically. We may unlock new treatments, grow replacement organs, or even reverse aspects of aging. The future of medicine may well depend on mastering the science of stem cells.
But with great power comes great responsibility. We must navigate the ethical, social, and scientific challenges with care, ensuring that stem cell technologies serve humanity with integrity and compassion.