Biologically important molecules undergo dramatic structural changes when exposed to ultraviolet (UV) radiation, a phenomenon that is well-known but not entirely understood, especially when it comes to some pharmaceutical compounds. The effects of UV radiation on such molecules are crucial for understanding a wide range of biological and medical processes, including skin cancer. To unravel these complexities, an international research team has employed an innovative technique that combines cutting-edge X-ray light with Coulomb explosion imaging. This novel method allows scientists to visualize the ultra-fast molecular changes that occur at the femtosecond timescale—a fraction of a second too brief for the human eye to detect. The results of this groundbreaking study were recently published in the prestigious journal Nature Communications.
The Role of 2-Thiouracil in Medical Research
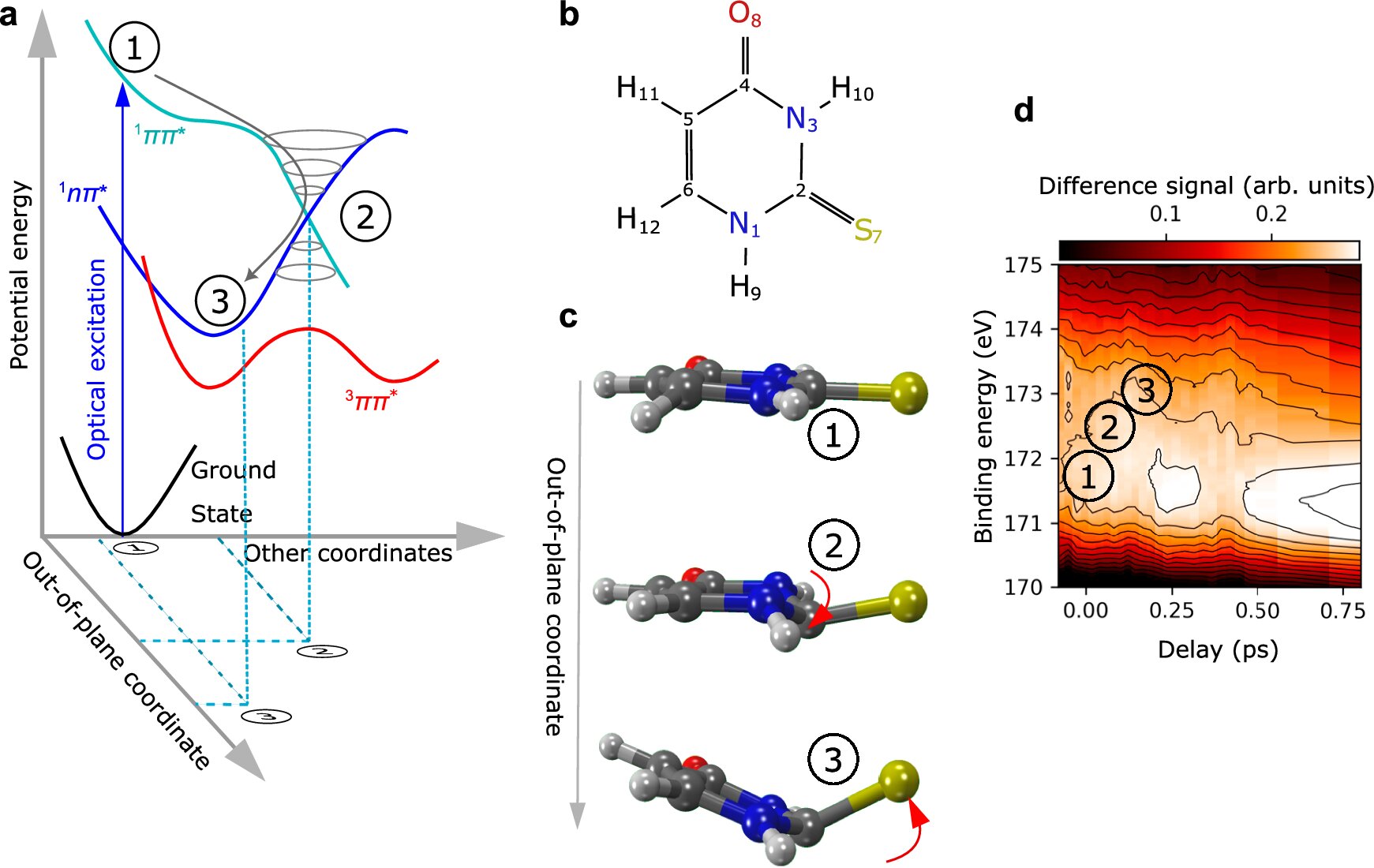
The molecule at the center of this research is 2-thiouracil, a sulfur-containing compound that is part of a class of pharmaceutically active substances. These molecules are similar to nucleobases—the building blocks of DNA. However, the addition of sulfur in 2-thiouracil imparts unique chemical and biological properties, making it of particular interest in medical research. 2-thiouracil is chemically related to substances that are essential for many biological processes, such as DNA synthesis and repair. Yet, unlike its DNA counterparts, the sulfur atom in 2-thiouracil makes the molecule exceptionally reactive when exposed to UV radiation.
Markus Gühr, the head of DESY’s free-electron laser FLASH and a professor at the University of Hamburg, is the study’s lead author. According to Gühr, “2-thiouracil and its related molecules become dangerously reactive when exposed to UV radiation.” This heightened reactivity poses serious health risks, such as an increased potential for skin cancer, which is thought to be linked to the molecule’s unstable and reactive nature when it absorbs UV light.
The Challenge of Understanding Ultra-Fast Molecular Changes
When 2-thiouracil absorbs UV radiation, its molecular structure undergoes rapid changes that can last only a few femtoseconds (1 femtosecond = 1 trillionth of a second). These changes can lead to the molecule becoming highly reactive, yet traditional methods have struggled to capture these processes. This challenge prompted the research team to utilize an advanced technique known as Coulomb explosion imaging.
Coulomb explosion imaging works by bombarding a molecule with intense X-ray pulses. This intense radiation knocks electrons out of the molecule, resulting in a positive charge that destabilizes the molecule. The charged molecule, now in an unstable state, rapidly breaks apart into various fragments. By tracking the direction in which these fragments fly apart, scientists can deduce the molecular structure of the compound and how it changes under different conditions.

In earlier studies, Coulomb explosion imaging was primarily used for simpler molecules. However, this new study marks a significant leap forward, as the team successfully applied this technique to 2-thiouracil, a more complex and biologically relevant molecule.
Technical Innovation: Combining X-ray Light and Slow-Motion Imaging
The research team, including scientists from Goethe University Frankfurt, the European XFEL in Schenefeld, and the Deutschen Elektronen-Synchrotron DESY in Hamburg, developed a revolutionary experimental setup. The use of the world’s most powerful X-ray laser—the European XFEL—enabled the researchers to generate incredibly short and intense X-ray pulses. These pulses were essential for initiating the Coulomb explosion process and for observing the molecule’s rapid transformations.
The setup also involved a two-pulse process. An additional UV pulse was applied just before the X-ray pulse to excite the molecules and induce their structural changes. By varying the time between the two pulses, the team was able to capture a slow-motion movie of the changes occurring in the molecule. This approach allows the team to observe events that take place in the blink of an eye—within 100 to 1000 femtoseconds—offering an unprecedented view into the dynamic world of molecular reactivity.
As Till Jahnke, the study’s first author and a professor at Goethe University Frankfurt, explains: “By varying the time interval between the UV and X-ray pulses, we can obtain a slow-motion movie of these ultra-fast processes.” Jahnke’s expertise in experimental atomic and molecular physics was pivotal in this breakthrough. His team used the SQS (Small Quantum Systems) scientific instrument at the European XFEL to apply Coulomb explosion imaging to a biologically relevant molecule for the first time, expanding the experimental possibilities of this technique beyond fundamental physics research.
Insights into Molecular Behavior and Reactivity
The results of this experiment yielded two groundbreaking findings. The first relates to the structural transformation of 2-thiouracil when exposed to UV radiation. Under UV exposure, the molecule, which is typically flat in its normal state, undergoes a dramatic bending. This bending causes the sulfur atom to protrude from the molecular structure, creating a highly reactive intermediate state. This newly formed structure is not only unstable but also long-lived, enabling the molecule to engage in chemical reactions that could lead to damage in biological tissues—such as in the case of skin cancer.
This finding stands in stark contrast to typical DNA nucleobases, which do not contain sulfur. As Gühr explains, “Unlike 2-thiouracil, regular nucleobases have mechanisms to deal with UV radiation by converting the absorbed energy into harmless heat through various excitation states.” The sulfur atom in 2-thiouracil prevents this protective mechanism from working, leading to its increased reactivity when exposed to UV radiation.
The second key discovery was related to the experimental technique itself. The team realized that they did not need to track every atom in the molecule to reconstruct its structure. By focusing on measuring only a few key atoms—such as the sulfur, oxygen, and hydrogen atoms—the team was able to gather enough data to reconstruct the molecule’s changes. The six carbon atoms in 2-thiouracil were not essential for this analysis, simplifying the experimental process and opening the door for more efficient measurements on even more complex molecules in future studies.
Future Implications of the Research
This study’s findings not only provide valuable insights into the reactivity of molecules like 2-thiouracil but also mark a significant advancement in molecular imaging. The ability to observe ultra-fast molecular processes in slow motion has vast potential for a wide range of scientific fields, including chemistry, biology, and pharmacology. In particular, this method could be used to explore the mechanisms of drug action, molecular damage caused by radiation, and the interactions between different biomolecules.
By applying Coulomb explosion imaging to more complex and biologically relevant molecules, researchers can now analyze how these molecules respond to various stimuli, such as radiation or chemical exposure, at an unprecedented level of detail. This breakthrough could lead to the development of better pharmaceutical treatments, more effective radiation therapies, and enhanced cancer prevention strategies.
Conclusion
The research published in Nature Communications offers a glimpse into a future where the dynamic processes of molecules are visible in slow motion. The ability to visualize ultra-fast structural changes at the atomic level opens new avenues for scientific exploration and innovation. By using a combination of X-ray light and Coulomb explosion imaging, the team has not only expanded our understanding of 2-thiouracil but has also pioneered a method that can be applied to a wide variety of biologically important molecules. This breakthrough may eventually lead to new strategies in drug development, cancer prevention, and molecular biology, further cementing the importance of cutting-edge technologies like X-ray lasers in advancing scientific knowledge.
Reference: Till Jahnke et al, Direct observation of ultrafast symmetry reduction during internal conversion of 2-thiouracil using Coulomb explosion imaging, Nature Communications (2025). DOI: 10.1038/s41467-025-57083-3