Deep within the body, there are battles fought in silence. Some of the most ruthless involve cancers so embedded, so clever in their evasiveness, that even the most powerful treatments bounce off them like stones off armor. Pancreatic cancer. Brain tumors. Metastatic melanoma. These are among the “cold” tumors—the ones that seem immune to immunotherapy, resistant to chemotherapy, and unreachable by the body’s own defense systems.
For decades, scientists have sought a way to smuggle potent treatments directly into the heart of these tumors, to turn the tide of war from within. And now, a new study, published in Science Translational Medicine, offers one of the most promising answers yet.
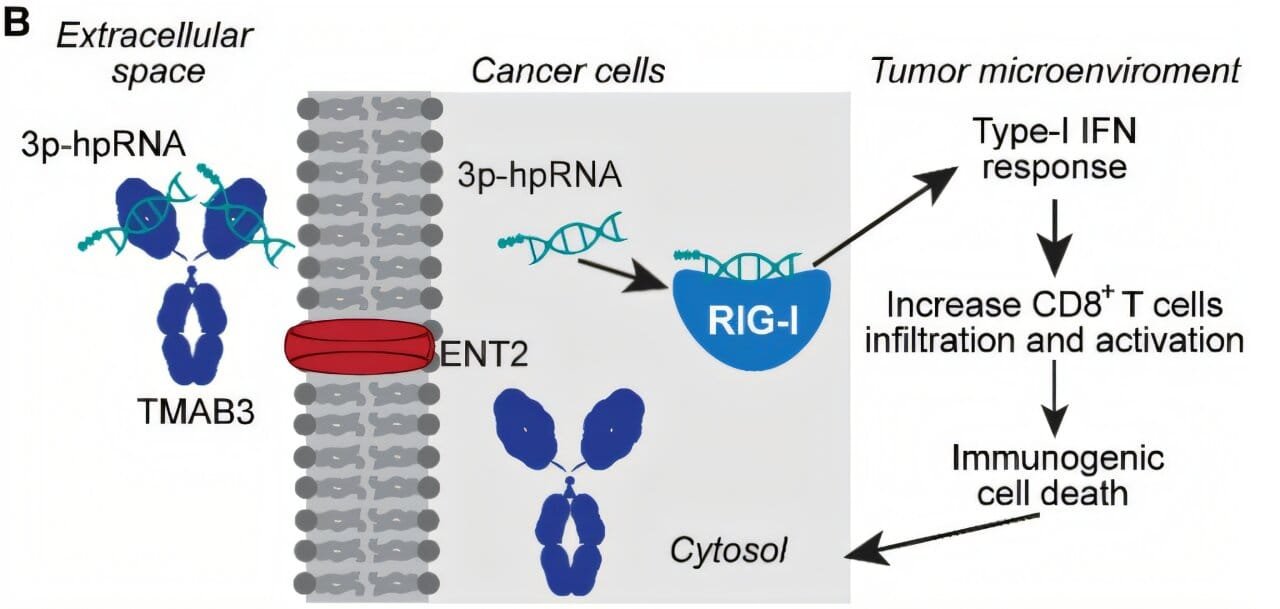
In a breakthrough that feels part precision engineering and part biological espionage, researchers at Yale School of Medicine have developed a modified antibody—dubbed TMAB3—that can deliver RNA therapies precisely to these hard-to-reach tumors. In animal models, it didn’t just find its mark—it slashed tumor size and extended survival, all while sparing healthy tissues from collateral damage.
Cracking the Code of RNA Delivery
RNA therapy has long been hailed as the future of medicine. Unlike conventional drugs, which often bluntly interfere with protein production, RNA can be tailored to fine-tune genetic instructions themselves. It’s the blueprint-level intervention that could theoretically stop cancer cells from multiplying, hijack their signaling, or even make them more visible to the immune system.
But here’s the catch: RNA is fragile. And getting it inside a tumor is like trying to deliver a message into a fortress surrounded by guards, gates, and landmines. The immune system often treats RNA as a virus, attacking it on sight. And when delivered systemically, most RNA never reaches its target. Instead, it’s degraded in the bloodstream, filtered out by the liver, or scattered harmlessly across healthy tissue.
Enter TMAB3—a specially engineered antibody designed to bind RNA like a molecular chaperone and smuggle it directly to the tumor.
“Our finding that TMAB3 can form antibody/RNA complexes capable of delivering RNA payloads to tumors provides a new approach to overcome this challenge,” says Dr. Peter Glazer, senior author and professor of therapeutic radiology and genetics at Yale.
This was not just a case of attaching RNA to any available antibody and hoping for the best. The team used computer modeling to redesign TMAB3 so it could securely bind to a therapeutic RNA molecule known to stimulate innate immune responses. They then humanized the antibody—a process that makes it less likely to be flagged as a foreign invader by the immune system. It was, in short, purpose-built for stealth and specificity.
The Enemy Within: Targeting Cold Tumors
The real test came when this bioengineered duo was set loose in animal models carrying three of the most notorious cancers: pancreatic ductal adenocarcinoma, medulloblastoma (a pediatric brain cancer), and melanoma (a deadly skin cancer that often spreads aggressively).
These are tumors that don’t respond well to today’s best treatments. They hide from the immune system, trigger little inflammation, and often lie beyond natural barriers—like the blood-brain barrier that protects the brain from most drugs. They are, in many ways, tumors designed to survive.
Yet TMAB3, armed with its RNA payload, was able to penetrate even these strongholds.
In the pancreatic cancer model, tumors shrank dramatically. More importantly, survival was extended, thanks to a boost in CD8+ T cells—the immune system’s elite assassins, now activated and aimed at the cancer.
In the medulloblastoma models, the results were equally stunning. The therapy crossed into the brain, reached the tumors, shrank them, and did it all without triggering harmful immune responses in healthy tissue. The precision wasn’t just surgical—it was molecular.
And in melanoma, which can be particularly brutal when it metastasizes, the treatment stifled tumor growth without toxicity. No significant side effects. No widespread inflammation. Just targeted elimination.
A Platform for the Future of Oncology
What’s most exciting about this innovation isn’t just the performance—it’s the potential. TMAB3 isn’t a single-use bullet. It’s a platform. It’s modular. Which means future therapies could load different kinds of RNA payloads—each designed to trigger a specific immune or genetic reaction in a different type of tumor or patient.
“With further development, this platform could support personalized immuno-RNA therapies,” says Dr. Luisa Escobar-Hoyos, senior author and associate professor of therapeutic radiology and molecular biophysics and biochemistry at Yale. “By achieving targeted delivery to tumor cells without systemic toxicity, we open the possibility of developing treatments that are not only tumor-specific but also adaptable to the immunologic context of each patient’s cancer.”
That last phrase—“the immunologic context of each patient’s cancer”—is key. It’s a recognition that cancer is not one disease, but many, and even within the same type, no two tumors behave the same. The future of treatment is not generic; it’s tailored.
And with TMAB3, scientists may have built the tool that finally allows RNA therapies to be used in that personalized, safe, and effective way.
Beyond the Lab: Toward Human Trials
Of course, the road from animal models to human treatment is long, complex, and uncertain. There are regulatory hurdles, safety trials, manufacturing logistics, and the ever-present challenge of scaling a laboratory success into a global solution. But the groundwork laid by the Yale team is unusually robust.
The antibody has been humanized, a critical first step for future human trials. The RNA cargo has already shown effectiveness in stimulating innate immune responses—a mechanism increasingly recognized as vital in rallying the immune system against cancer. And the fact that no major toxicities were observed in animal models bodes well for eventual clinical translation.
“We’re not just talking about another tool in the toolbox,” says one of the researchers off-record. “We’re talking about rewriting the playbook—bringing RNA therapy into solid tumors where it’s never been able to go before.”
Smuggling Messages of Hope
In many ways, TMAB3 isn’t just carrying RNA. It’s carrying hope—not the abstract kind, but the practical, biochemical kind that lives inside syringes, protocols, and translational science.
It’s the kind of hope that imagines a child with brain cancer surviving because their treatment could reach past the blood-brain barrier without causing brain inflammation.
It’s the kind of hope that envisions a pancreatic cancer patient—someone who might otherwise face months, not years—gaining more time, better time, because a treatment finally reached the tumor hiding in plain sight.
It’s the kind of hope built molecule by molecule, from the dreams of scientists who refuse to give up on the hard cancers, the forgotten patients, the cases that don’t respond.
There’s still much to do. Clinical trials must be designed. More variations of RNA payloads must be tested. Safety must be confirmed again and again. But for the first time, a bridge has been built—one that crosses not only the barriers within the body, but the historical barriers that have kept RNA therapies from reaching their full potential.
And at the center of it all is a single, elegant molecule, repurposed and redesigned, sneaking life-saving instructions past the enemy’s gates.
Reference: Elias Quijano et al, Systemic administration of an RNA binding and cell-penetrating antibody targets therapeutic RNA to multiple mouse models of cancer, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adk1868