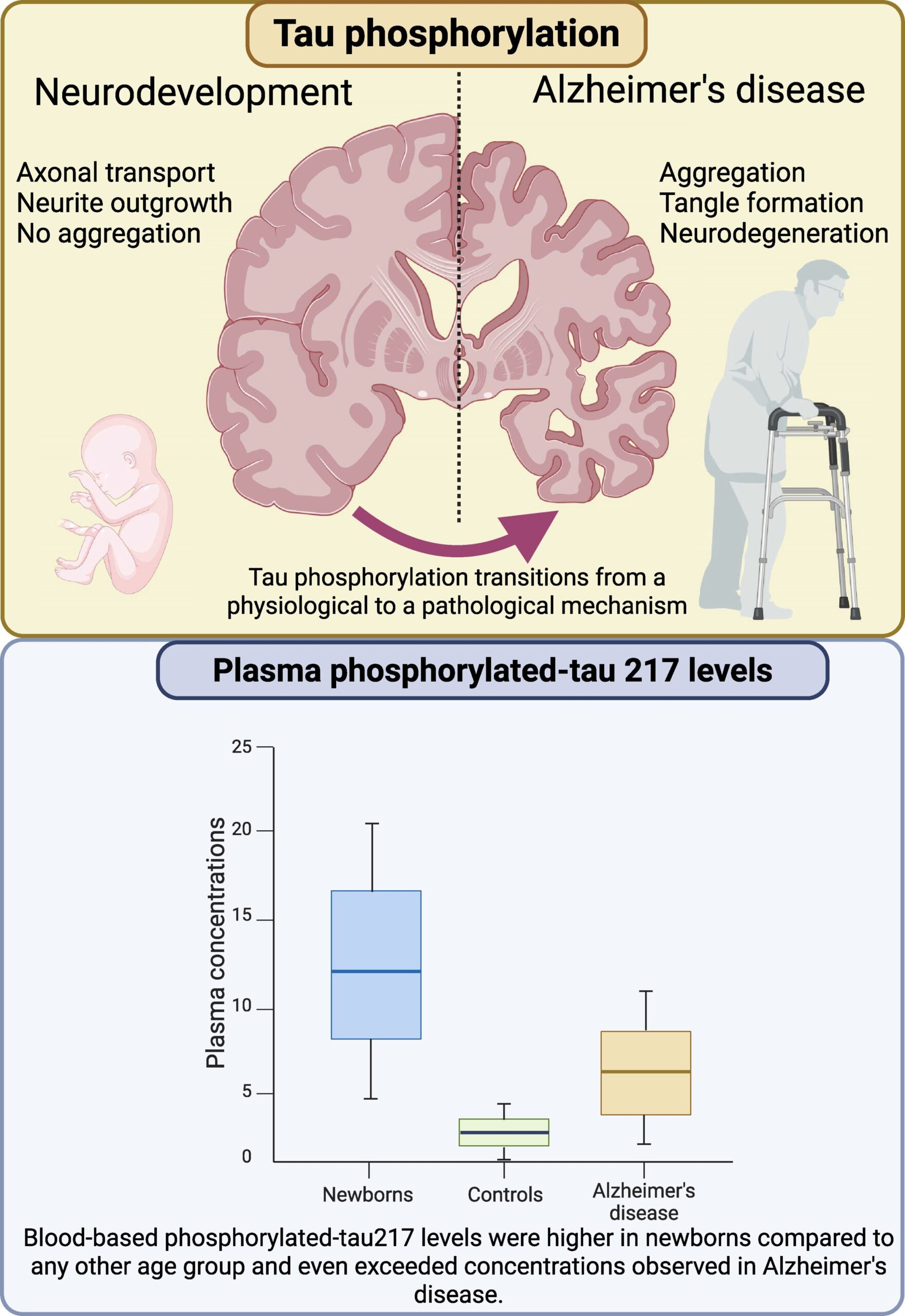
It sounds like the setup to a riddle: what do the brains of newborn babies and people with Alzheimer’s disease have in common?
At first glance, almost nothing. One is a brain just beginning to form memories, connections, and awareness. The other is a brain unraveling, its architecture collapsing under the weight of a degenerative disease. And yet, as a new international study reveals, both share a striking biological signature: elevated levels of a protein called p-tau217, a hallmark used to diagnose Alzheimer’s disease.
In a discovery that’s turning heads in the neuroscience community, researchers from the University of Gothenburg, alongside colleagues in Spain and Australia, have reported that newborn infants have even higher blood levels of p-tau217 than Alzheimer’s patients—a finding that challenges existing assumptions and opens a window into the earliest and latest chapters of brain life.
The study, published in Brain Communications, could reshape how we understand brain development and neurodegeneration, and might even offer clues for future Alzheimer’s treatments.
The Alzheimer’s Marker That Showed Up in Newborns
For years, phosphorylated tau (p-tau217) has been viewed through a single lens: a red flag. In the brains of people with Alzheimer’s, tau proteins misfold and clump into tangles—disrupting neurons, killing brain cells, and contributing to memory loss and cognitive decline. Rising p-tau217 in the blood has therefore been hailed as one of the most promising diagnostic biomarkers for identifying Alzheimer’s early, even before symptoms appear.
But the new study found something unexpected.
Analyzing blood samples from more than 400 people, including healthy newborns, premature infants, young adults, older adults, and Alzheimer’s patients, the team discovered that the highest levels of p-tau217 weren’t in elderly patients—but in babies only days old. Premature infants had especially elevated levels, which then dropped steadily over the first few months of life, stabilizing around adult levels later in infancy.
“This was completely unexpected,” said Dr. Fernando Gonzalez-Ortiz, first author of the study. “We thought of p-tau217 as a signal of disease. But here it is, at astonishing levels, in the healthiest brains possible.”
A Protein With Two Faces
Why would a protein linked to destruction play such a dominant role in the construction of the brain?
The answer lies in context. While p-tau217 is associated with chaos in aging brains, in newborns, it may be a master builder.
Previous animal studies had hinted that tau proteins are involved in early brain development, but this is the first time scientists have measured p-tau217 directly in human infant blood, confirming its presence and magnitude. In infants, rather than contributing to tangles and cell death, p-tau217 appears to be helping neurons grow, differentiate, and form connections—the foundation of brain architecture.
It’s a biological paradox. The same molecule that marks a brain in decline may be crucial for building one.
“In Alzheimer’s, tau aggregates and gums up the machinery of thought. But in babies, tau may be helping lay down the very circuits that allow thought to happen,” said Professor Kaj Blennow, the study’s senior author and a pioneer in Alzheimer’s biomarker research.
Timing Is Everything
One of the most compelling findings of the study is the link between p-tau217 levels and gestational age. The earlier a baby was born, the higher their p-tau217 concentration. This suggests that the protein may help compensate for the challenges of early delivery, speeding up brain development during a critical window when neurons are racing to catch up.
This idea—that p-tau217 is not only present but also functional during early development—turns long-standing beliefs about tau biology on their head.
It also raises an intriguing question: if newborn brains can safely handle such high levels of phosphorylated tau, why do older brains falter when much smaller amounts begin to accumulate?
A Potential Key to Future Therapies
The idea that the brain once knew how to manage p-tau217 without harm opens a provocative new frontier in Alzheimer’s research. What if we could reactivate those protective mechanisms in the aging brain?
“We believe the newborn brain holds secrets,” Gonzalez-Ortiz said. “It can tolerate high tau, even thrive with it. If we can understand what protects it, we might find new ways to intervene in Alzheimer’s—not just by targeting tau directly, but by copying how the young brain controls it.”
The implications extend beyond basic science. Plasma p-tau217 recently received FDA approval as a diagnostic tool for Alzheimer’s, which means clinicians are already using this marker to detect disease. But this new study suggests that elevated tau isn’t always a sign of pathology. The context, timing, and biology of the brain matter.
“If we don’t understand why p-tau217 is elevated,” Blennow warned, “we risk misinterpreting the signal. Especially in research trials or in future treatments, we need to know whether we’re looking at a sign of disease—or of something else entirely.”
Rewriting the Story of Tau
This study marks a turning point in our understanding of tau proteins and brain development. It suggests that p-tau217 isn’t simply a molecular villain, but a dynamic player with different roles across the human lifespan. Its appearance in the blood of newborns—at levels even Alzheimer’s patients don’t reach—tells us the story of tau is far from simple.
It is a story that begins in the earliest days of life, as neurons burst into being, and continues in the slow unfolding of age and memory. Somewhere between those extremes, the delicate balance of tau tips. If we can understand why—and how to prevent the fall—we may not only learn how to diagnose Alzheimer’s earlier, but how to rewrite its ending.
Until then, a newborn’s blood may be the most unexpected place to look for hope.
Reference: Fernando Gonzalez-Ortiz et al, The potential dual role of tau phosphorylation: plasma phosphorylated-tau217 in newborns and Alzheimer’s disease, Brain Communications (2025). DOI: 10.1093/braincomms/fcaf221