In the heart of one of the nation’s most storied psychiatry programs, a transformative shift is quietly underway—one that may alter not just how we understand a once-feared treatment, but also how we view the brain itself. At the center of this shift is Dr. Zach Rosenthal, MD, PhD, a third-year psychiatry resident at the University of Pennsylvania, who, like many great clinicians before him, found his calling not in textbooks but in the delicate drama of human suffering and recovery.
During his psychiatry clerkship in medical school, Rosenthal encountered a case that would ignite a lifelong inquiry. A young man with schizophrenia had developed severe catatonia—a neuropsychiatric state so profound that he lay motionless in bed, unable to speak or eat. He was adrift in a terrifying neurological limbo, unresponsive to medications, and slipping dangerously toward medical crisis. That’s when Rosenthal’s team turned to electroconvulsive therapy, or ECT—a procedure many still associate with outdated and stigmatized portrayals, but which, in the right hands, can be nothing short of life-saving.
“After a single ECT treatment,” Rosenthal recalled, “he could speak in full sentences and move well enough to feed himself. After a few more treatments, he walked out of the hospital and returned to living independently.” That moment, part miracle and part mystery, seeded the question that still drives Rosenthal’s work: Why does ECT work so well when nothing else does?
ECT Reimagined: From Cultural Relic to Cutting-Edge Science
For much of the 20th century, ECT carried the baggage of its past. First introduced in 1938, it quickly gained notoriety—not for its efficacy, which was clear even then—but for its often crude administration and the trauma that patients endured in its early years. Popular culture didn’t help: films like One Flew Over the Cuckoo’s Nest embedded in the public consciousness the idea of ECT as punishment rather than treatment.
Yet the reality today is radically different. ECT is now administered under general anesthesia with muscle relaxants, carefully monitored by teams of psychiatrists, anesthesiologists, and nurses. It remains the gold standard treatment for severe, treatment-resistant depression, bipolar mania, catatonia, and some forms of psychosis. And its safety profile, especially in expert hands, is remarkably strong.
Still, the central mystery lingers. For all its clinical success, ECT’s mechanism of action has remained largely theoretical. The long-standing assumption has been that the induced seizure—a controlled burst of synchronized neuronal firing—is responsible for the therapeutic effect. But as Rosenthal and his collaborators have discovered, that might only be part of the story.
Cracking the Neural Code: A Second, Hidden Event
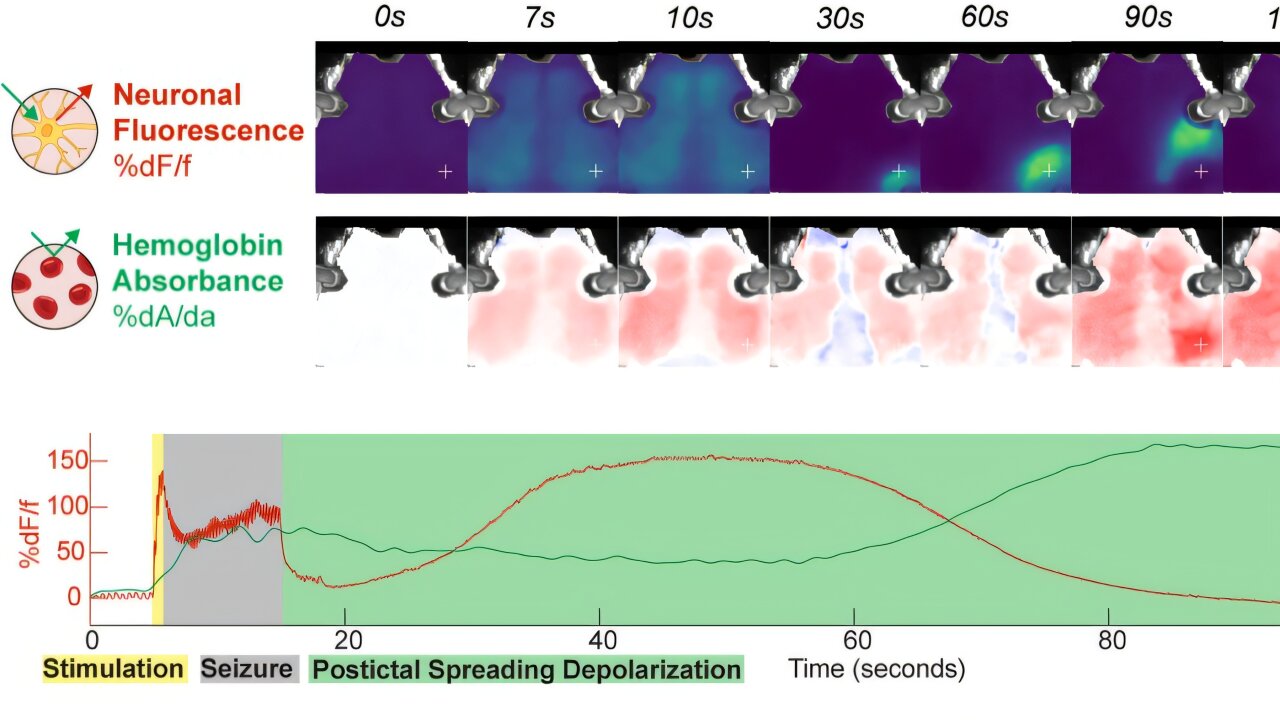
In a recent study published in Nature Communications, Rosenthal and his interdisciplinary team uncovered something entirely new—a second, massive brain event that occurs immediately after the ECT-induced seizure: cortical spreading depolarization, or CSD.
CSD is a slow-moving, high-amplitude wave of neuronal depolarization that sweeps across the brain like a rolling blackout, silencing virtually every neuron in its path. First observed in animal models decades ago, CSD has been implicated in conditions like migraine aura and stroke. But it had never before been detected after ECT—not in over 80 years of clinical use. The reason? It required the invention of entirely new tools to see it.
Using optical neuroimaging—a non-invasive technique developed by physicists at Penn that uses light to measure brain activity—the team was able to visualize CSD in both mouse models and human patients post-ECT. “It’s a kind of hard reset,” Rosenthal explained. “It shuts everything down and allows the brain to reboot. This could be the key to understanding why ECT works where other treatments fail.”
Toward Precision Psychiatry: ECT in the Age of Biomarkers
The discovery of CSD in ECT-treated brains is more than just a scientific curiosity; it represents a potential paradigm shift. If the seizure is merely the trigger and CSD the real therapeutic event, then clinicians could someday tailor ECT to maximize the induction of CSD while minimizing unwanted side effects.
That’s the vision Rosenthal and his colleagues are now pursuing. “We’ve known for years that the quality of the seizure—its duration, intensity, and location—matters for treatment outcomes. But we didn’t know why,” he said. “Now we’re using tools like optical neuroimaging, computational modeling, and functional MRI to map CSD and determine its relationship to symptom improvement.”
This effort is now part of a broader collaboration with Dr. Yvette Sheline, a nationally recognized expert in neuromodulation and Director of Penn’s Center for Neuromodulation in Depression and Stress (CNDS). Their joint clinical trial is designed to test the provocative hypothesis that it’s not the seizure per se, but the inhibition caused by CSD, that mediates ECT’s therapeutic effects.
“This gives us the opportunity to understand the mechanism of ECT in a radically new way,” Sheline said. “We are hopeful that this model will help us not only improve treatment for current patients but also extend the reach of ECT to new populations.”
A New Frontier for an Old Therapy
The implications of this work stretch far beyond academic curiosity. If CSD is confirmed as a reliable biomarker for treatment response, it could revolutionize the way ECT is administered. Current dosing is often based on broad clinical guidelines and adjusted through trial and error. But a biomarker-guided approach—one rooted in real-time monitoring of brain activity—could make ECT more precise, effective, and individualized.
Imagine a future where a psychiatrist can visualize a patient’s brain response during treatment and adjust stimulation parameters in real-time, targeting just the right areas and intensities to induce CSD without excessive cognitive side effects. This is no longer science fiction—it’s an active area of research that Penn and its partners are helping bring to life.
Even more tantalizing is the possibility of using this knowledge to develop entirely new treatments. If CSD is itself therapeutic, could we someday develop non-seizure-based methods to induce it? Could medications, transcranial magnetic stimulation, or other neuromodulatory techniques be tailored to harness its benefits? “These are the questions we’re starting to ask,” Rosenthal said. “And the answers could transform how we treat mental illness.”
Beyond the Science: The Power of Human Stories
What makes Rosenthal’s journey especially compelling is that it’s rooted in the messy, poignant reality of patient care. His scientific questions didn’t emerge from the ivory tower—they were born in a hospital room, watching a young man frozen by catatonia come back to life.
Those stories are often lost in the public discourse around psychiatry and ECT. But they matter. They are the reason this research exists, and the reason it must continue. Every data point in Rosenthal’s study represents a person—someone who could not move, speak, or feel, and who, through a misunderstood treatment, found their way back to life.
“We have decades of evidence showing that ECT is safe and effective, but the stigma remains,” Rosenthal said. “Popular media rarely shows the reality: that this treatment, when done properly, can be life-saving. The stigma of ECT—and mental illness more broadly—has real consequences. It prevents people from accessing care that could change or save their lives.”
The Village Behind the Vision
Rosenthal is quick to point out that this work is the product of a vast collaborative effort. His mentors include Dr. Ethan Goldberg, a pediatric neurologist at Children’s Hospital of Philadelphia, and Dr. Mario Cristancho, who leads Penn’s ECT clinical team. Optical neuroimaging technology was developed by physicist Dr. Arjun Yodh and his lab, bringing a cross-disciplinary lens that bridges psychiatry, neurology, and physics.
“It really took a village,” Rosenthal said. “No one person could have done this. It’s a testament to what can happen when different fields come together around a shared question.”
Rewriting the Narrative of Mental Health Treatment
Ultimately, Rosenthal’s work is part of a broader movement to demystify and destigmatize psychiatry. It’s about bringing mental health treatment into the 21st century—not just through new tools, but by rewriting the stories we tell about them.
ECT is not an antiquated relic. It’s a modern, evolving intervention with deep roots in clinical science and new branches growing into the most advanced realms of neuroscience. The discovery of CSD may well be a milestone in psychiatry’s long journey from trial-and-error to precision medicine.
But more than anything, this work is a reminder of what’s at stake. In the right hands, with the right knowledge, ECT can give people their lives back. For the patients lying still in hospital beds, caught in the grip of disorders that resist everything else, the brain’s quiet “hard reset” might be the loudest hope of all.
Reference: Zachary P. Rosenthal et al, Electroconvulsive therapy generates a postictal wave of spreading depolarization in mice and humans, Nature Communications (2025). DOI: 10.1038/s41467-025-59900-1