In the silent depths of our bodies, far beyond conscious thought or sensation, microscopic armies are always at war—some to harm, others to heal. In this invisible battlefield, researchers from the National Cancer Center Research Institute in Tokyo have uncovered a surprising new ally: a previously unknown gut bacterium named Hominenteromicrobium strain YB328.
Though barely measurable by the eye, this bacterium appears to carry immense potential. By awakening key immune cells and guiding them into tumors, YB328 is changing how scientists think about the relationship between the gut and the immune system. Even more strikingly, patients whose microbiomes harbor this strain experience longer progression-free survival when treated with immune checkpoint inhibitors.
In a world where cancer immunotherapy is both a beacon of hope and a puzzle yet unsolved, YB328 could be a critical missing piece.
Immunotherapy’s Quiet Revolution—and Its Unfinished Business
Over the last decade, immune checkpoint blockade (ICB) therapies have transformed cancer care. These therapies work by lifting the biological “brakes” that cancer places on immune cells, especially T cells. By targeting PD-1 or PD-L1—molecules that tumors use to disguise themselves—monoclonal antibodies can restore the immune system’s ability to recognize and destroy cancerous cells.
For many patients, these treatments offer the first real shot at long-term remission. But success is uneven. Some respond dramatically. Others don’t respond at all.
The great mystery has been why.
Over time, scientists began suspecting the answer might lie not just in the tumor or the immune system—but in the gut. It turned out that the trillions of microbes inside the human digestive tract play a critical role in shaping immunity. Different bacteria send different signals. Some encourage inflammation. Others promote healing. And some—like YB328—may prime the immune system for battle in a way no drug alone can.
Finding a Microbial Signature in Human Hope
The new study, published in Nature and led by Japanese researchers, began with a question: could a specific bacterium explain why some patients fare better with PD-1 blockade therapy?
To find out, the team prospectively collected stool samples from 50 Japanese patients undergoing immune checkpoint therapy—15 with non-small cell lung cancer and 35 with gastric cancer. Each of these individuals had received anti-PD-1 monoclonal antibodies, but with widely varying results.
Using 16S rRNA gene sequencing, the researchers profiled the bacterial makeup of each patient’s gut. They then conducted advanced statistical analyses—including receiver operating characteristic (ROC) curves and principal coordinate analysis—to identify patterns that distinguished responders from non-responders.
Among all the microbial candidates, one stood out.
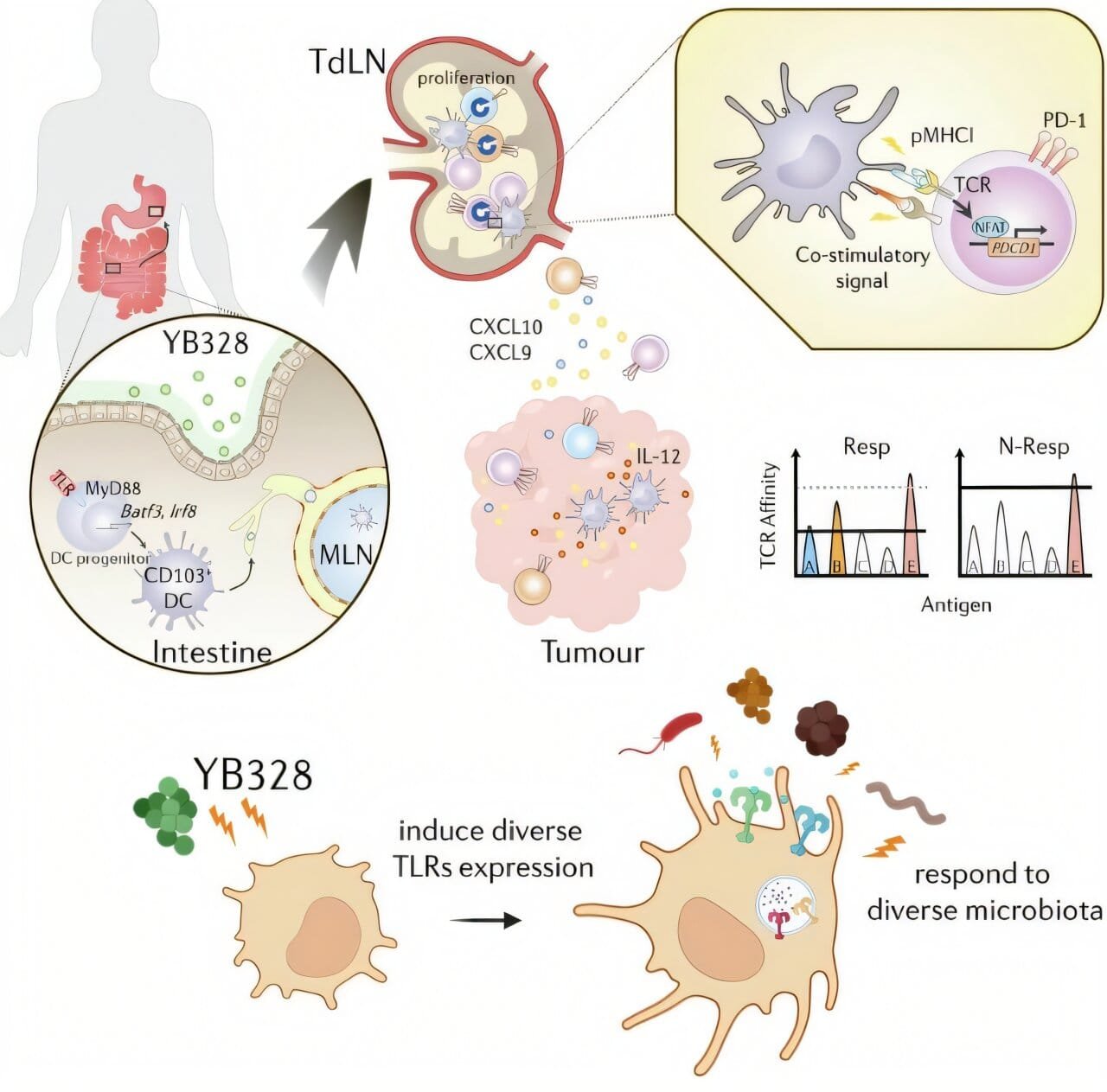
Strain YB328, a previously undescribed member of the family Ruminococcaceae, was found almost exclusively in those patients who responded well to therapy. It was not just present—it was dominant.
Turning Gut Bacteria into Immune Weapons
Finding a correlation is one thing. Proving causation is another. To do that, the scientists turned to mice.
Using fecal microbiota transplantation, they introduced gut bacteria from human responders—those who carried YB328—into germ-free mice. The mice were then implanted with tumors and treated with anti-PD-1 therapy.
The result was astonishing.
Mice colonized with YB328-rich microbiota showed significantly slower tumor growth, longer survival, and more robust immune activity in tumors. There was a measurable increase in activated CD8+ T cells, the elite assassins of the immune system. Even more compelling, these T cells showed signs of higher diversity and functionality. They produced more cytokines and carried a wider range of T cell receptors—suggesting a broader capacity to recognize and kill tumor cells.
This wasn’t just random noise. It was a symphony of immune activation, and YB328 was the conductor.
Dendritic Cells: The Secret Signal Boosters
Behind this microbial-driven immune awakening lay another set of players: dendritic cells.
Often described as the sentinels of the immune system, dendritic cells patrol the body, capturing fragments of pathogens or tumors and presenting them to T cells like wanted posters. Without dendritic cells, T cells would have no targets and no guidance.
In YB328-treated mice, tumors showed an unusually high number of CD103+ CD11b− conventional dendritic cells. These are precisely the kind needed to activate CD8+ T cells against cancer. The data revealed that YB328 was not merely enhancing general inflammation—it was orchestrating a highly specific immune strategy.
In vitro experiments confirmed this. Dendritic cells exposed to YB328 upregulated co-stimulatory molecules like CD86, CD80, and MHC-I, turning them into highly efficient T cell activators. When these dendritic cells encountered naïve CD8+ T cells, they promoted greater expansion, stronger PD-1 expression (a marker of activation), and heightened recognition of tumor-associated antigens.
In essence, YB328 was transforming the immune system into a more precise, more effective weapon—by pushing the right buttons in the gut.
The Danger of the Wrong Microbes
Not all gut bacteria are helpful. Some seem to do the opposite.
In the same study, researchers also examined the effects of Prevotella vulgatus, a bacterium previously associated with poor responses to immunotherapy. When P. vulgatus was introduced into the mice, it blunted the beneficial effects of YB328. Tumors grew faster. CD8+ T cell infiltration dropped. The immune system’s firepower was dampened.
This interaction emphasized the delicate balance of the gut ecosystem. It’s not simply a matter of adding the right bacteria. One must also remove or control the wrong ones.
Interestingly, some microbial genera previously believed to be beneficial—including Faecalibacterium, Bifidobacterium, and Akkermansia—showed no significant difference between responders and non-responders in this cohort. This underscores the need for population-specific studies and highlights how microbial effects can vary by geography, genetics, and diet.
Toward Personalized, Microbiome-Driven Cancer Therapy
The implications of this study are enormous.
First, YB328 may serve as a predictive biomarker, helping oncologists determine in advance who is most likely to benefit from PD-1 blockade therapy. This would allow for more precise, more effective cancer treatment strategies—and could spare patients from ineffective therapies.
Second, YB328 or its derivatives could become a therapeutic probiotic. Imagine a pill containing a carefully cultured strain of YB328, taken in conjunction with immunotherapy to improve outcomes. Unlike chemotherapy or even antibodies, such a treatment would be biologically harmonious, leveraging nature’s own symbiosis to enhance healing.
Third, this research adds weight to the idea that the gut microbiome should be considered a clinical organ—one that can be shaped, manipulated, and optimized as part of cancer care. With microbiome analysis and engineering, we could one day tailor treatments not just to a patient’s tumor profile, but to the ecology of their gut.
A Microscopic Discovery with Human-Sized Impact
In the end, YB328 is more than just a bacterium. It’s a window into the future of medicine—a future where treatment doesn’t only mean radiation, knives, or chemicals, but also cultivating the delicate gardens inside us.
The immune system is an ancient force, honed by millions of years of evolution. But it does not work alone. As this new research shows, its power can be dramatically amplified by a single microscopic companion.
This discovery reminds us that sometimes, the key to solving the most intractable human problems can be found in the most unexpected places—hidden in the soil of our own biology, waiting patiently to be seen.
And in the fight against cancer, YB328 has just stepped forward as a tiny hero in a very big war.
Reference: Nina Yi-Tzu Lin et al, Microbiota-driven antitumour immunity mediated by dendritic cell migration, Nature (2025). DOI: 10.1038/s41586-025-09249-8