For most of us, a night of four or five hours of sleep is a recipe for grogginess, irritability, and sluggish performance the next day. Over time, such chronic sleep deprivation can erode health, impair memory and concentration, weaken the immune system, and even elevate the risk of stroke, heart disease, and depression. Yet, there exists a rare group of people who, contrary to the medical consensus, function perfectly on just four to six hours of sleep—and not just function, but thrive. These individuals are not fueled by caffeine or willpower. They are natural short sleepers (NSS), biologically wired to need less sleep. Now, thanks to a groundbreaking study published in the Proceedings of the National Academy of Sciences, scientists have uncovered a crucial genetic mutation that may explain how this rare ability works.
The Science Behind Sleep: More Than Rest
Although sleep appears to be a state of inactivity, it is, in fact, a critical period of biological maintenance. As we sleep, our brains undergo neural reorganization, sorting memories and clearing waste proteins that accumulate during wakefulness. Meanwhile, the body repairs cells, balances hormones, regulates metabolism, and resets numerous systems that keep us functioning optimally. That’s why mainstream sleep science recommends 7–9 hours of sleep per night for adults.
Falling short of this benchmark can have a snowball effect on health. Sleep deprivation compromises decision-making, reaction time, and emotional regulation. In the long term, it raises susceptibility to chronic illnesses like type 2 diabetes, cardiovascular disease, obesity, and even neurodegenerative conditions like Alzheimer’s. And yet, natural short sleepers seem immune to these consequences, living long, healthy lives with far less shuteye than the average person. How?
A Genetic Mutation Unlocks the Mystery
Previous research has hinted at genetic underpinnings for NSS behavior, with mutations discovered in genes like DEC2, ADRB1, NPSR1, and GRM1. These rare mutations disrupt normal sleep-wake regulation, enabling people to maintain peak cognitive and physical performance with much less rest. But how these genes functioned and interconnected remained a complex puzzle.
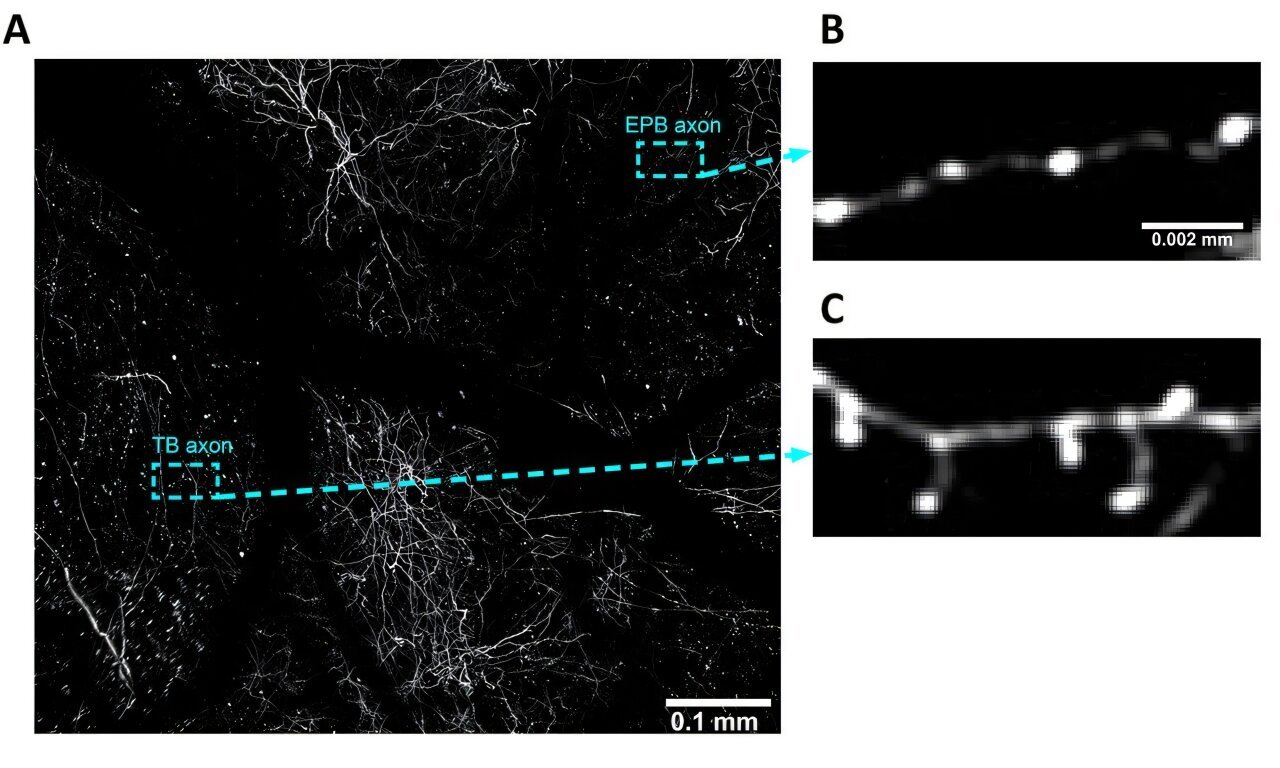
In the latest study, researchers zoomed in on a new player: salt-inducible kinase 3 (SIK3), a gene involved in cellular signaling pathways that regulate the body’s biological clock. Intracellular protein kinases like SIK3 are known to facilitate sleep by transferring phosphate groups to target proteins—an essential process for maintaining circadian rhythm and sleep depth. The team zeroed in on a variant of this gene, SIK3-N783Y, which had never been directly linked to natural short sleep until now.
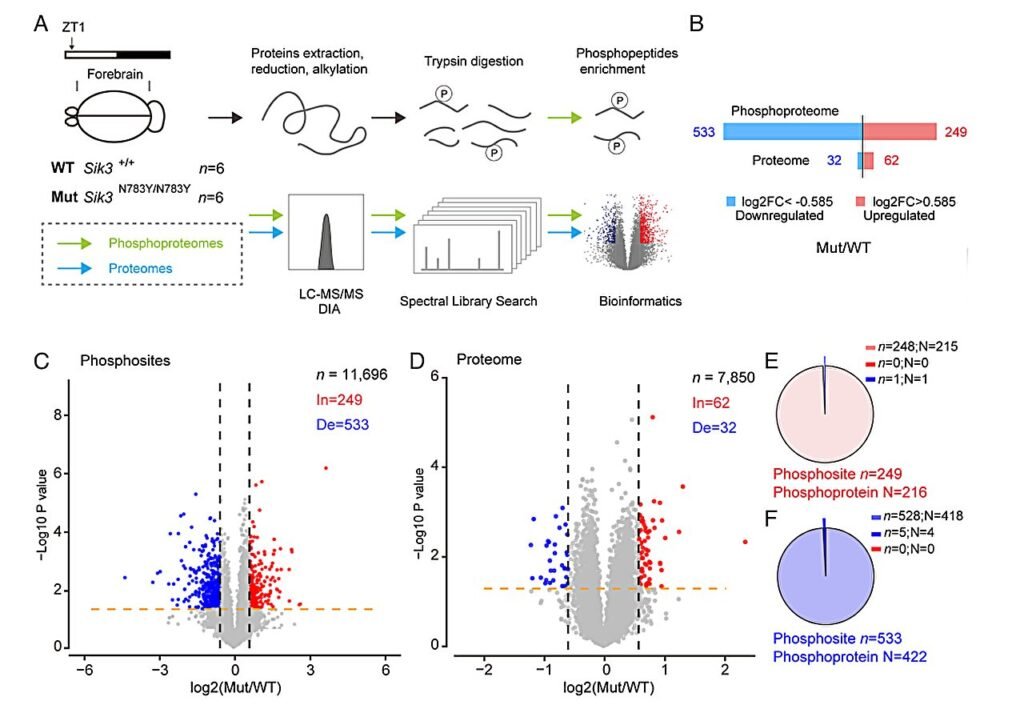
Meet the Sleeper: A 70-Year-Old Mystery
The investigation began with a remarkably healthy 70-year-old woman who reported sleeping only three hours a night—far less than the average adult—and had done so for most of her life. Yet, she exhibited none of the cognitive decline, metabolic issues, or emotional fatigue commonly associated with sleep deprivation. In fact, she led an exceptionally active life, free of chronic illness or mental deterioration.
To understand her physiology, researchers collected data on her sleep-wake cycle using wrist actigraphy—a non-invasive method of monitoring rest and activity patterns. Interestingly, the data revealed that while she reported sleeping only three hours, the actual average was about 6.3 hours per night, still considerably below the standard recommendation.
Decoding the Genetic Blueprint
The team then conducted whole-exome sequencing of her DNA to search for rare variants that might explain her NSS phenotype. What they found was compelling: a point mutation in the SIK3 gene that swapped a single amino acid in the protein structure—specifically, the substitution of asparagine (N) with tyrosine (Y) at position 783. This N783Y mutation was the key focus.
But was this mutation truly responsible for her short sleep trait? To test their theory, the scientists used CRISPR-Cas9 gene editing to introduce the same mutation into a line of laboratory mice. The results were striking. The genetically modified mice slept 30 minutes less than their non-mutated counterparts. In mouse terms, this is a significant reduction in total sleep time.
Structural Consequences at the Molecular Level
Digging deeper, computational modeling of the SIK3 protein revealed that the N783Y mutation significantly altered its three-dimensional structure. These structural changes hampered the protein’s ability to perform its essential function—phosphorylation of downstream targets in sleep-regulating pathways. In simpler terms, the mutant protein couldn’t properly “flip the biochemical switches” that tell the body to stay asleep longer or achieve deeper sleep.
As a result, sleep was not only shorter in duration but shallower in depth. Yet, neither the human subject nor the engineered mice exhibited the negative cognitive or physiological outcomes typically associated with such sleep reduction. It seems that this particular mutation changes the sleep architecture in a way that preserves the essential functions of sleep within a compressed time frame.
Implications for the Science of Sleep
This discovery of SIK3-N783Y as a genetic determinant of the NSS trait doesn’t just help explain a fascinating quirk of human biology. It reshapes our understanding of sleep itself. For decades, scientists have debated whether the 8-hour rule is a universal biological necessity or simply an average. Findings like this suggest that sleep need is, at least in part, genetically personalized. It also raises intriguing possibilities about how we might one day tailor treatments for sleep disorders.
Understanding how NSS individuals maintain healthy physiology on limited sleep could lead to new strategies for treating insomnia, sleep apnea, and even shift work disorder. If researchers can replicate or modulate the effects of the SIK3 mutation pharmacologically, we might one day develop therapies that allow people to get the benefits of full sleep in less time—without adverse health effects.
Evolutionary Insights and Biological Conservation
Perhaps even more fascinating is what the study suggests about evolutionary biology. The SIK3 gene is conserved across multiple species, from humans to rodents, underscoring its central role in sleep regulation. The fact that a single-point mutation can have such a profound effect on sleep patterns in both mice and humans suggests that the pathways governing sleep are deeply rooted in our genetic makeup.
This also opens doors to exploring whether other species in the animal kingdom exhibit similar mutations and sleep efficiency, offering a broader comparative framework for understanding sleep evolution. Could the genes of migratory birds, nocturnal predators, or hibernating mammals reveal more such “short-sleep hacks” written into DNA?
A New Frontier in Personalized Sleep Medicine
As scientists continue to identify and catalog genetic variations associated with sleep behaviors, we inch closer to a future where personalized sleep medicine becomes a reality. Imagine undergoing a genetic test that determines your optimal sleep duration and architecture. No more one-size-fits-all sleep prescriptions—just customized strategies based on your unique biology.
Moreover, such discoveries can inform public health campaigns, workplace policies, and mental health practices. Not everyone needs the same amount of sleep to be healthy, productive, and mentally resilient. Recognizing and respecting individual variability can reduce stigma and improve quality of life, especially for those who may have been misdiagnosed or misunderstood.
Conclusion: Rethinking Sleep Through the Lens of Genetics
The SIK3-N783Y mutation is more than just a genetic curiosity—it is a window into the intricate, finely tuned mechanisms that govern one of the most essential yet least understood aspects of our biology. It reminds us that while sleep is universal, our need for it is not. For natural short sleepers, evolution may have crafted an efficient rest strategy that defies conventional wisdom. For the rest of us, the journey to better sleep may one day involve more than pillows and melatonin—it may lie in the code written in our genes.
By unlocking the biological blueprint behind NSS, researchers are paving the way for smarter, more effective sleep interventions. And perhaps, one day, we may all be able to enjoy the benefits of a full night’s sleep—in half the time.
Reference: Hongmin Chen et al, The SIK3-N783Y mutation is associated with the human natural short sleep trait, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2500356122