Nearly 13.8 billion years ago, our universe was born in a cataclysmic event known as the Big Bang—a moment of incomprehensible energy and heat. In the seconds that followed, matter began to take shape from pure energy. The first building blocks of everything we see today emerged: protons, neutrons, and electrons. But this was still an unimaginably hostile environment, a roiling plasma where even simple atoms couldn’t yet exist.
As the universe expanded, it cooled. Within minutes, protons and neutrons fused into the first atomic nuclei, mainly hydrogen and helium. Yet these young nuclei remained bare, stripped of their electrons by the intense heat. It would take another 380,000 years—a blink of an eye in cosmic terms—for temperatures to drop enough for electrons to finally bind to these nuclei, forming the first neutral atoms.
This pivotal moment, called recombination, transformed the universe from an opaque plasma into a transparent cosmic ocean. It marked the end of the earliest chaos and opened the door to chemistry itself.
The Birth of the First Molecule
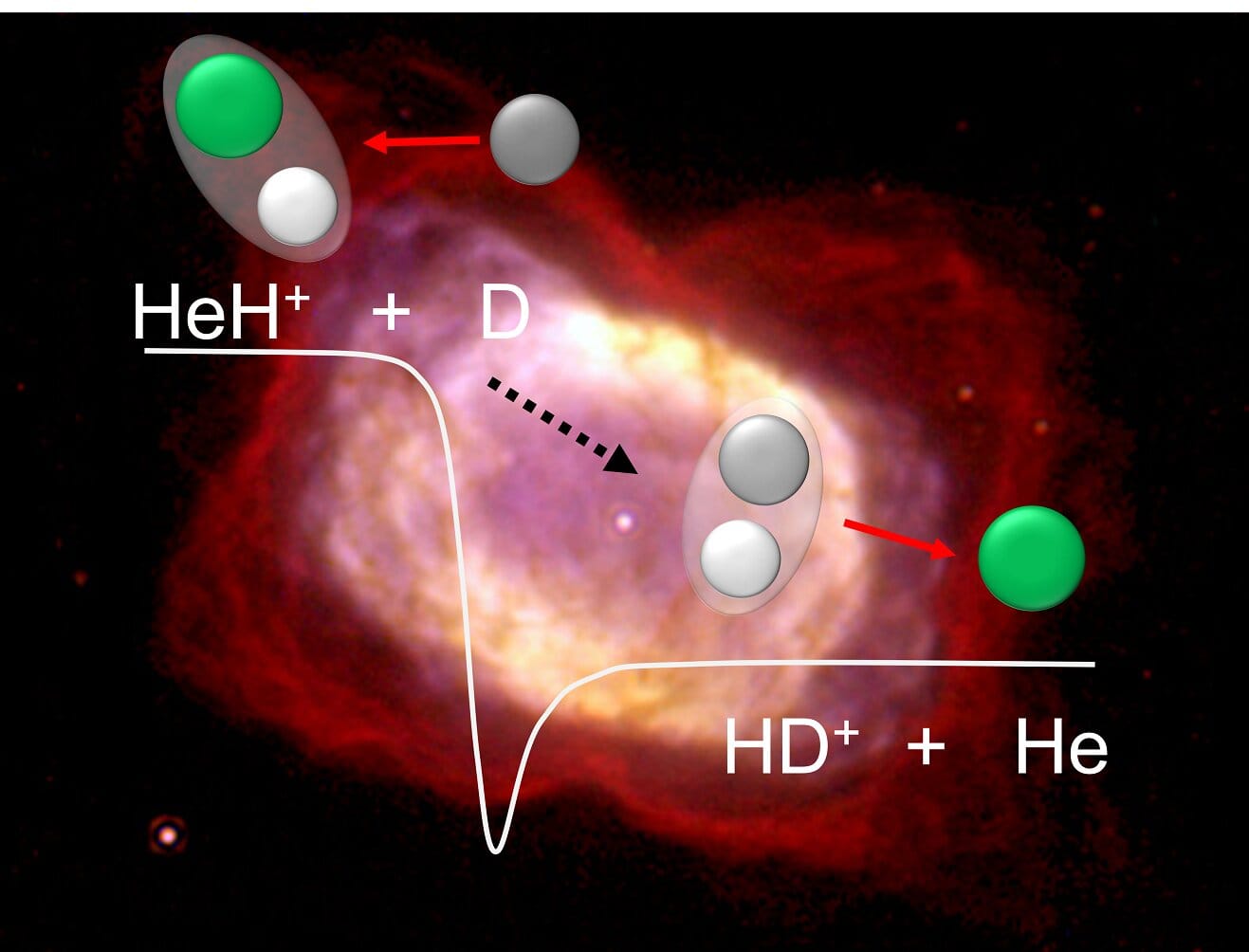
Out of this cooling primordial soup, a humble molecular pioneer emerged: helium hydride (HeH⁺). This fragile ion was born when a neutral helium atom bonded with an ionized hydrogen nucleus. Though simple, HeH⁺ holds monumental significance—it was the very first molecule to ever exist in the universe.
This tiny molecular bond sparked the first steps of cosmic chemistry. HeH⁺ helped trigger a cascade of reactions that would eventually produce molecular hydrogen (H₂), the most abundant molecule in the cosmos. This molecular evolution wasn’t just chemical curiosity—it played a crucial role in shaping the universe’s first stars.
Darkness Before the Light
After recombination, the cosmos entered a long, silent epoch known as the cosmic dark ages. There were no stars, no galaxies—only vast clouds of hydrogen and helium drifting through darkness. To ignite the first stars, these gas clouds needed to collapse under gravity, becoming dense enough for nuclear fusion to begin.
But gravity alone wasn’t enough. As these early protostellar clouds contracted, they heated up. To collapse fully, they had to shed this heat. At high temperatures, collisions between atoms could release energy as photons, cooling the gas. Yet below about 10,000°C, this mechanism faltered. The universe needed molecules—capable of emitting energy through vibrations and rotations—to cool further.
Helium hydride, with its strong dipole moment, was perfectly suited for this task. Acting as a cosmic coolant, it allowed primordial gas clouds to lose energy efficiently, setting the stage for molecular hydrogen to form. This, in turn, enabled gravity to pull matter tightly enough to spark the first stellar furnaces. Without HeH⁺ and H₂, the first stars might never have been born.
The Fragile Chemistry of the Early Universe
However, helium hydride didn’t last long in its original form. In the young universe, it frequently collided with free hydrogen atoms, leading to its own destruction. These interactions produced molecular hydrogen ions (H₂⁺), which quickly transformed into neutral H₂ molecules and a proton. In this way, helium hydride was both a chemical pioneer and a stepping stone—briefly existing, yet catalyzing the molecular chain reaction essential for cosmic evolution.
Recreating Ancient Chemistry on Earth
For decades, astrophysicists theorized about helium hydride’s role in the early cosmos. Yet, without experimental confirmation, its chemical behavior remained partly speculative. Now, researchers at the Max-Planck-Institut für Kernphysik (MPIK) in Heidelberg have recreated this primordial chemistry under space-like conditions for the first time.
Using the Cryogenic Storage Ring (CSR)—a unique 35-meter-diameter ion storage ring cooled to just a few kelvins (-267°C)—scientists stored helium hydride ions and collided them with beams of deuterium atoms, an isotope of hydrogen containing an extra neutron. In these ultracold, high-vacuum conditions, researchers could mimic the delicate chemistry of the early universe.
What they found surprised them. Contrary to long-standing predictions, the reaction rate between helium hydride ions and neutral hydrogen (or deuterium) did not decrease at lower temperatures. Instead, it remained almost constant—a discovery that upends previous theoretical models.
Dr. Holger Kreckel of MPIK explained, “Earlier calculations suggested that as the universe cooled, this reaction would slow dramatically. But our experiment shows it stayed highly efficient even at the lowest cosmic temperatures. This means helium hydride was far more chemically active than we believed.”
A Theoretical Puzzle Solved
In parallel with the experiments, a team of theoretical physicists led by Yohann Scribano revisited decades-old calculations describing the potential energy surface of this reaction. They uncovered a fundamental error that had misled scientists for years. With corrected models, theoretical predictions now align closely with the experimental data, strengthening confidence in the results.
This breakthrough has profound implications: it suggests that helium hydride played an even greater role in driving molecular hydrogen formation than previously understood. That means the early universe may have been primed for star formation much sooner than astrophysicists once thought.
Illuminating the First Stars
The formation of molecular hydrogen was critical for enabling primordial gas clouds to cool and collapse into the first stars. These stars, hundreds of times more massive than our Sun, burned hot and bright, synthesizing heavier elements in their cores and eventually exploding as supernovae.
Their light ended the cosmic dark ages, reionizing the universe and laying the chemical foundation for galaxies, planets, and eventually life itself. Without helium hydride quietly catalyzing the early chemistry of the cosmos, this stellar dawn might have been delayed—or might never have come at all.
A Glimpse into Our Origins
The MPIK experiment doesn’t just answer a question about exotic cosmic chemistry—it connects us to our deepest origins. Every atom in your body, every molecule of water, every star in the night sky owes its existence to these first molecular bonds forged in darkness.
By recreating helium hydride’s ancient reactions, scientists have taken a step closer to understanding how a universe of light and complexity emerged from the featureless simplicity of the Big Bang.
In this sense, helium hydride is more than the universe’s first molecule—it’s a silent architect of creation, a fleeting chemical bridge that allowed gravity and time to sculpt the cosmos we know today.
Reference: F. Grussie et al, Experimental confirmation of barrierless reactions between HeH⁺ and deuterium atoms suggests a lower abundance of the first molecules at very high redshifts, Astronomy & Astrophysics (2025). DOI: 10.1051/0004-6361/202555316