For decades, scientists believed they understood a sobering truth about the ocean: that essential trace metals like iron and zinc—so vital for sustaining life—sink into the abyss of deep-sea sediments and disappear forever. In this vision, the nutrients that feed microscopic algae at the ocean’s sunlit surface fell into darkness and decay, never to return. But a new study from ETH Zurich is flipping that narrative on its head.
Beneath the waves, in the cold and crushing silence of the deep ocean floor, a hidden alchemy is unfolding. There, deep within marine sediments, metals that were thought to be lost forever are not gone at all. They are leaking back into the ocean in a quiet, crucial process that sustains the life cycles of the sea—and helps regulate the planet’s climate.
“This changes how we view ocean chemistry, and its impact on ocean biology and climate,” says Derek Vance, the ETH Zurich geochemist who led the groundbreaking study published in Nature.
Microscopic Algae, Massive Impact
At the surface of the ocean, where sunlight penetrates only about 100 meters down, lives an invisible engine that drives Earth’s climate. These are the phytoplankton—microscopic algae that drift on currents and soak up sunlight to photosynthesize, just like plants on land. They pull carbon dioxide out of the atmosphere and use it to build their cells, contributing as much to global carbon fixation as the vast forests on land.
But unlike their terrestrial cousins, these algae do not root into soil. Instead, they rely on seawater to supply all the nutrients they need: nitrogen, phosphorus, and a delicate balance of trace metals like iron and zinc.
When they die, these plankton sink, taking with them the precious metals they consumed. In the deep ocean, their bodies decay, and the nutrients dissolve into the cold waters. From here, scientists have long believed, a slow and limited process begins—one where some of those nutrients may eventually return to the surface through ocean mixing, but many are thought to be lost forever, trapped in sediments miles beneath the surface.
A New Chapter in Ocean Chemistry
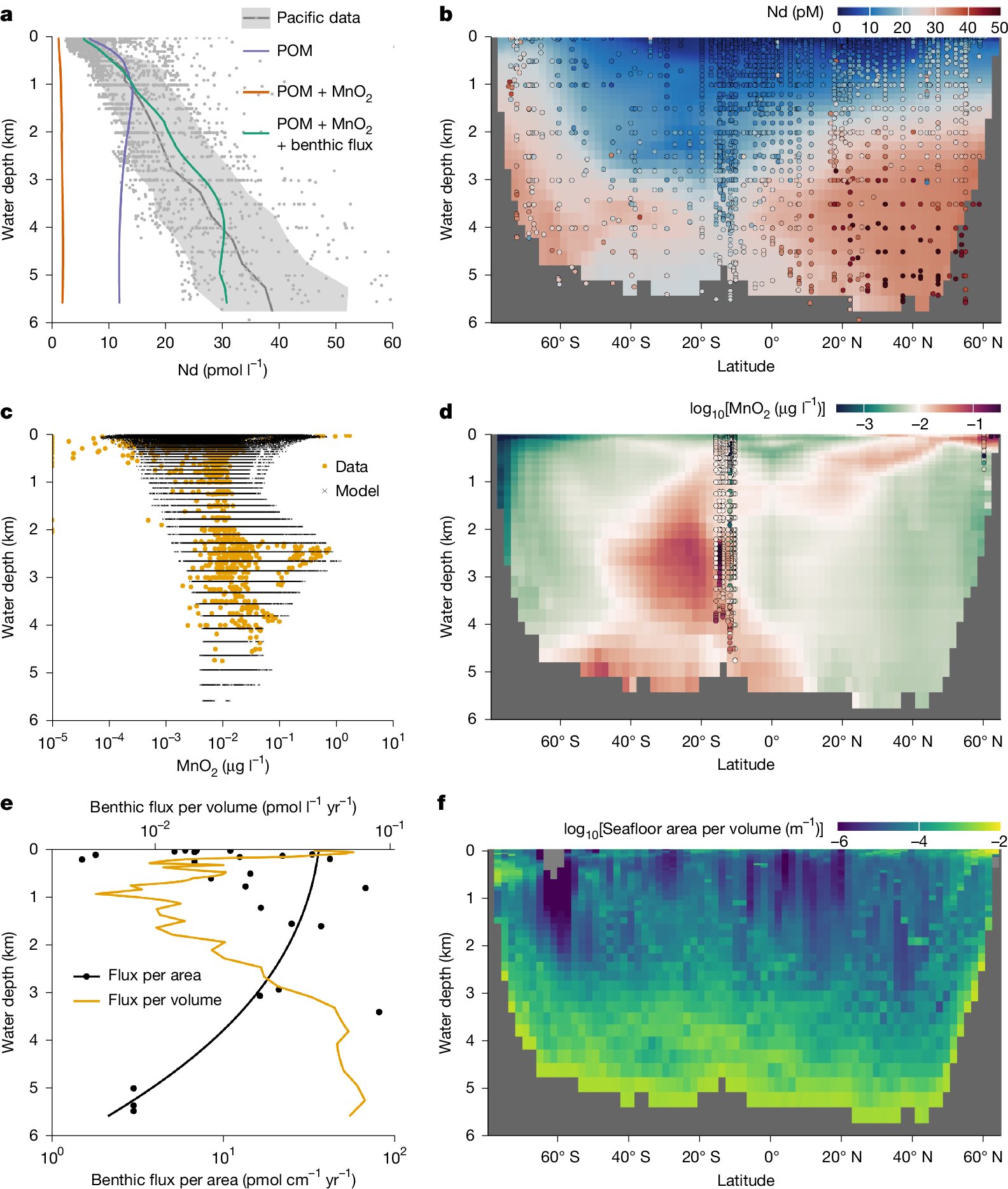
But Vance and his team have revealed a vital twist in this story. By using chemical tracers and high-resolution modeling, they discovered that many metals don’t vanish forever into sediments. Instead, after being trapped by manganese oxides—particles that rain down from seawater and sequester metals into solid form—these elements undergo a dramatic transformation.
Inside the sediment, far below the surface, chemical reactions break apart the manganese oxides, freeing the trace metals back into solution. This “leakage” flows from the sediment into the deepest waters of the ocean, where they begin a slow but steady journey back upward.
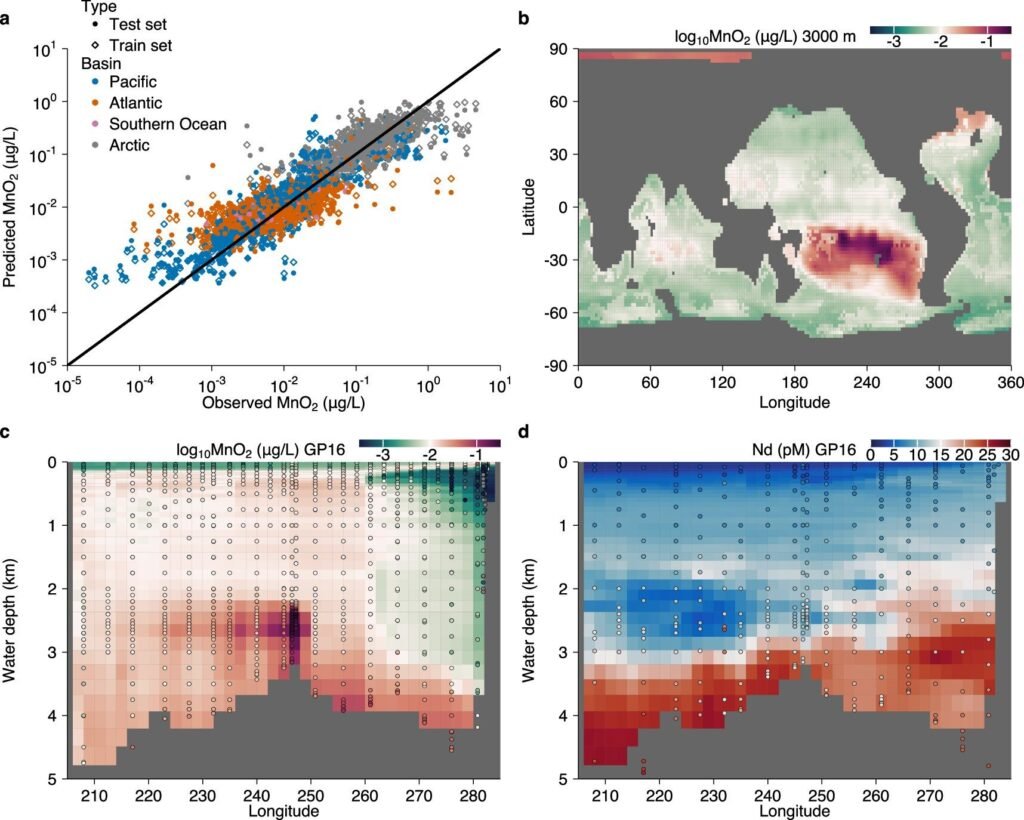
Using numerical models that simulate the physical transport of water through the ocean, the researchers confirmed that these recycled metals are indeed mixed back into the upper layers of the sea—where they can once again feed phytoplankton and fuel the biological engine of the planet.
“This is the first time we’ve been able to demonstrate this process in such detail,” Vance explains. “It means that the ocean has a kind of deep recycling system that we’ve vastly underestimated.”
The Secret Power of Trace Metals
Iron and zinc might not sound as important as carbon or nitrogen, but for phytoplankton, they’re make-or-break. These metals act as cofactors in vital enzymes that control photosynthesis and nitrogen fixation. Without them, the entire marine food web begins to falter.
And since phytoplankton play a pivotal role in pulling carbon from the atmosphere and locking it away in the deep ocean, these trace metals have far-reaching implications for Earth’s climate system.
That’s why the new findings matter so deeply—not just for marine biologists, but for climate scientists, policymakers, and anyone who seeks to understand how the planet breathes.
“The availability of trace metals governs the productivity of the oceans,” Vance says. “That, in turn, influences the levels of carbon dioxide in our atmosphere—past, present, and future.”
Rethinking the Deep Sea
For generations, scientists viewed the ocean floor as a graveyard for nutrients, a place where vital elements went to die. But the ETH Zurich team’s findings offer a radical reframe: the deep sea is not just an endpoint—it’s a crucible of regeneration.
By identifying sediment leakage as a major pathway for metal recycling, the research shifts long-held assumptions about ocean nutrient cycles and deepens our understanding of how the marine system regulates itself over time.
This new knowledge will likely change how ocean models simulate nutrient availability, biological productivity, and carbon drawdown—key variables in forecasting climate change scenarios.
Moreover, it raises pressing questions. How will changing ocean temperatures and chemistry affect these deep-sea reactions? Could warming waters alter the efficiency of this hidden recycling system? And what does this mean for geoengineering proposals that aim to enhance phytoplankton growth to capture carbon?
A Planet-Wide Implication From the Deep
The implications are global, and they run deep—literally. As humans search for ways to combat climate change, understanding the subtle feedback loops of the ocean becomes more vital than ever. Tiny elements like iron, buried kilometers below, may hold surprising influence over the climate dynamics that shape our future.
In that sense, Vance and his team haven’t just uncovered a new piece of geochemical knowledge. They’ve unearthed a quiet, powerful system that connects the abyssal depths of Earth’s oceans to the thin veil of atmosphere we all share.
Sometimes, the biggest revelations come not from the stars above—but from the sediments below.
Reference: Jianghui Du, Abyssal seafloor as a key driver of ocean trace-metal biogeochemical cycles, Nature (2025). DOI: 10.1038/s41586-025-09038-3. www.nature.com/articles/s41586-025-09038-3