Far beyond Earth’s pale blue sky, where stars shimmer like diamonds on black velvet, ice is more than just frozen water—it’s cosmic history, a vault of secrets waiting to thaw.
For decades, scientists believed that the ice scattered through the universe—blanketing comets, dusting the moons of distant planets, and floating in interstellar clouds—was utterly chaotic: an amorphous, structureless glass, frozen so fast it never had time to crystallize. But new research from University College London and the University of Cambridge has cracked that cosmic ice shell wide open.
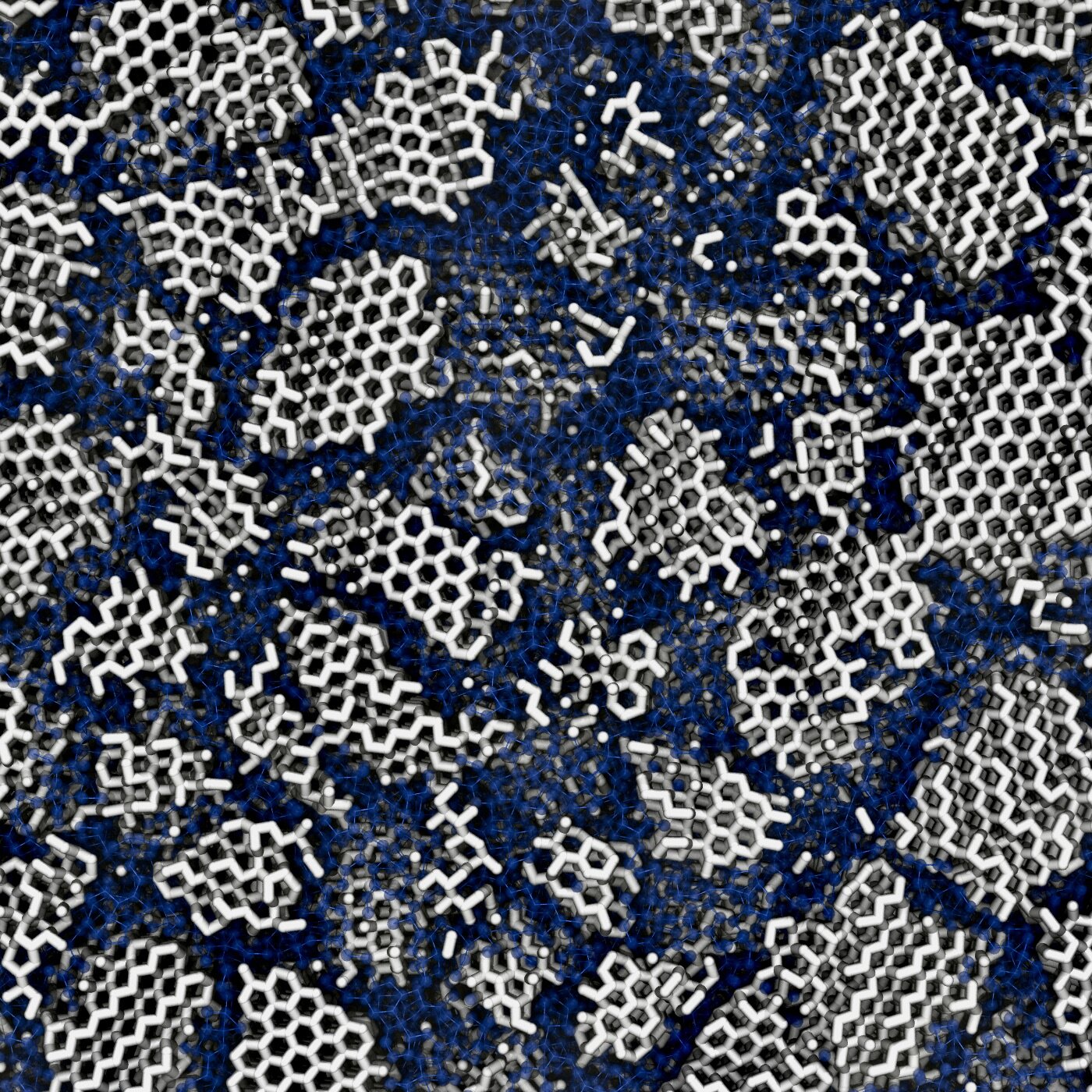
Hidden inside the icy darkness of space, it turns out, lie tiny, glimmering crystals—only a few billionths of a meter across—that change everything we thought we knew about water, the cosmos, and even the building blocks of life.
The Crystals Beneath the Chaos
When you imagine ice, you might think of snowflakes under a microscope: delicate, geometric marvels, each a unique sculpture of order. That’s the crystalline ice we know on Earth, shaped by relatively balmy temperatures and the gentle dance of water molecules slotting into precise, hexagonal lattices.
But in the bitter cold of space—where temperatures plunge to minus 120 degrees Celsius or lower—scientists long assumed ice was different. Lacking the energy to form those tidy crystal grids, water molecules were thought to freeze in a jumble, creating amorphous ice, a glassy solid more like frozen chaos than frozen water.
Yet, according to the new study published in Physical Review B, even this “space ice” carries hidden structure. Using advanced computer simulations alongside meticulous lab experiments, the team discovered that low-density amorphous ice—the most common form of ice in the universe—is not completely disordered after all. Instead, it contains minuscule crystalline domains: islands of order floating in a sea of randomness.
“These crystals are only about three nanometers wide—just a smidge larger than a strand of DNA,” said Dr. Michael B. Davies, lead author of the study, who conducted the research during his Ph.D. at UCL and Cambridge. “But they’re enough to give this cosmic ice a secret architecture we never suspected.”
Rewriting the Story of Cosmic Ice
To crack this icy mystery, the scientists built virtual boxes of water molecules and cooled them at different rates, simulating how water might freeze in interstellar space. Sometimes, as they cooled the simulations, they saw tiny crystals forming inside otherwise amorphous ice.
When they compared their virtual ice to real-world data from X-ray diffraction—a technique that maps how atoms are arranged by tracking how X-rays scatter—they found a striking match. The best agreement came from ice that was up to 20% crystalline, revealing that cosmic ice is a hybrid of chaos and order.
But the team didn’t stop in the realm of computer models. They also created actual samples of amorphous ice in the lab, mimicking how water freezes in the void. By gently warming these samples, they watched as their internal structure transformed into crystalline ice. Crucially, the exact pattern of crystals that emerged depended on how the ice had originally formed.
“If the ice were purely amorphous, it shouldn’t ‘remember’ anything about how it formed,” said co-author Professor Christoph Salzmann of UCL Chemistry. “But we saw clear differences, which hints that even amorphous ice contains crystalline regions.”
Ice, Life, and the Origins of Everything
These revelations have cosmic implications. Ice in space isn’t just frozen water; it’s an active player in the grand drama of the universe. It helps form planets, shapes comets, and shepherds vital molecules through interstellar space.
One of the most intriguing possibilities is whether ice could have been the delivery system for life’s raw materials on Earth. According to the panspermia hypothesis, comets laden with organic molecules might have seeded our planet with the building blocks of biology.
But the new findings could complicate that idea. “Our results suggest that if ice has a partly crystalline structure, there’s less empty space where organic molecules could hide,” said Dr. Davies. “That could make ice a less effective carrier for life’s building blocks.”
Still, there’s hope for panspermia enthusiasts. Even in partially crystalline ice, regions of disorder remain—pockets where complex molecules might still hitch a ride across light-years of emptiness.
A Window Into Water’s Weirdness
Beyond astrobiology, the study reshapes our understanding of water itself. Water is famously quirky—its molecules behave in ways that confound scientists, leading to bizarre properties like expanding when it freezes or having unusually high surface tension.
Professor Angelos Michaelides from the University of Cambridge believes the secrets of amorphous ice might hold clues to these mysteries. “Water is the foundation of life, but we still don’t fully understand it,” he said. “Amorphous ices may help explain some of water’s many anomalies.”
The implications stretch into human technology as well. Many high-tech materials—like the glass fibers that carry data through the internet—rely on being amorphous. If even those materials secretly harbor tiny crystals, it might affect how we design the technology of the future.
“If we can learn to remove these micro-crystals, we might improve the performance of fiber optics or other glass-based devices,” said Salzmann.
The Endless Quest to Understand Ice
Amorphous ice has been captivating scientists for almost a century. In the 1930s, researchers first discovered its low-density form by condensing water vapor onto metal surfaces chilled to -110 degrees Celsius. In the 1980s, they stumbled on its high-density cousin by squeezing ordinary ice under crushing pressure at icy temperatures.
Last year, the team behind the new study added a third kind: medium-density amorphous ice, eerily similar in density to liquid water itself. That discovery hinted that water might have multiple hidden solid states—each with different secrets.
And the quest continues. Could there be a truly amorphous ice, with not even a hint of crystals? Does the size of these nano-crystals vary depending on how cosmic ice forms?
“We’re just scratching the surface,” Davies said. “Every new piece of the puzzle reveals how much more there is to discover.”
So the next time you look up at the night sky, remember: the universe is laced with ice—ice that isn’t merely frozen water but a mysterious material, quietly recording the story of stars, planets, and possibly life itself. And within that frozen darkness, tiny crystals glitter, unseen, whispering secrets of a cosmos still waiting to be understood.