In 1984, when Israeli scientist Daniel Shechtman peered into the heart of a metallic alloy and spotted a pattern that seemed to defy the very laws of crystallography, he was laughed out of the room. What he claimed to see—atoms arranged with a five-fold symmetry, like twenty-sided dice stitched together—was considered physically impossible. And yet, there it was: a quasicrystal, neither random like glass nor repeating like traditional crystals, but something eerily in between.
Now, more than four decades later, scientists at the University of Michigan have done something equally bold. They’ve finally cracked the code behind these bizarre structures using a powerful new computational technique, offering the first quantum-mechanical evidence that quasicrystals aren’t freaks of nature—they’re stable, predictable, and governed by rules we’re just beginning to understand.
Published today in Nature Physics, the study changes what scientists thought they knew about solid matter. And it does so by venturing into a realm long considered out of reach: the quantum landscape of materials that refuse to play by the old rules.
The Quiet Revolution of a Forbidden Structure
To understand what makes quasicrystals so strange—and so revolutionary—you have to grasp what they’re not.
In conventional crystals like diamonds or table salt, atoms are arranged in a repeating, ordered lattice that extends uniformly in all directions. These patterns are predictable, beautiful, and fundamentally symmetric. They’re what materials scientists live by.
Glass, by contrast, is the wild child. Its atoms are frozen in place during rapid cooling, locking into a chaotic arrangement with no repeating structure at all. It’s disorder with a dash of elegance.
Quasicrystals sit between these two worlds. Like crystals, they are locally ordered—each atom fits neatly with its neighbors. But like glass, that order never repeats. Instead, their structures resemble the intricate tiling of Islamic mosaics or Penrose tiles, complex yet never redundant.
It was this non-repeating order that threw the scientific world into turmoil when Shechtman first observed it. Crystals, by definition, weren’t supposed to allow five-fold symmetry. That kind of pattern just didn’t tessellate—until quasicrystals came along and proved otherwise.
Shechtman’s discovery eventually earned him the Nobel Prize in Chemistry in 2011. But the physics community was still left scratching its head.
The Mystery of Stability
Why do quasicrystals form at all? What makes them stable when their atomic pattern lacks the repeating predictability that conventional theory depends on?
Until now, those questions were largely unanswerable. That’s because the go-to computational method for studying materials—density-functional theory—relies on repeating units to model atomic interactions and energy levels. No repeating units, no simulation.
“Quasicrystals have forced us to rethink how and why certain materials can form,” said Wenhao Sun, the study’s corresponding author and the Dow Early Career Assistant Professor of Materials Science and Engineering at the University of Michigan. “Until our study, it was unclear to scientists why they existed.”
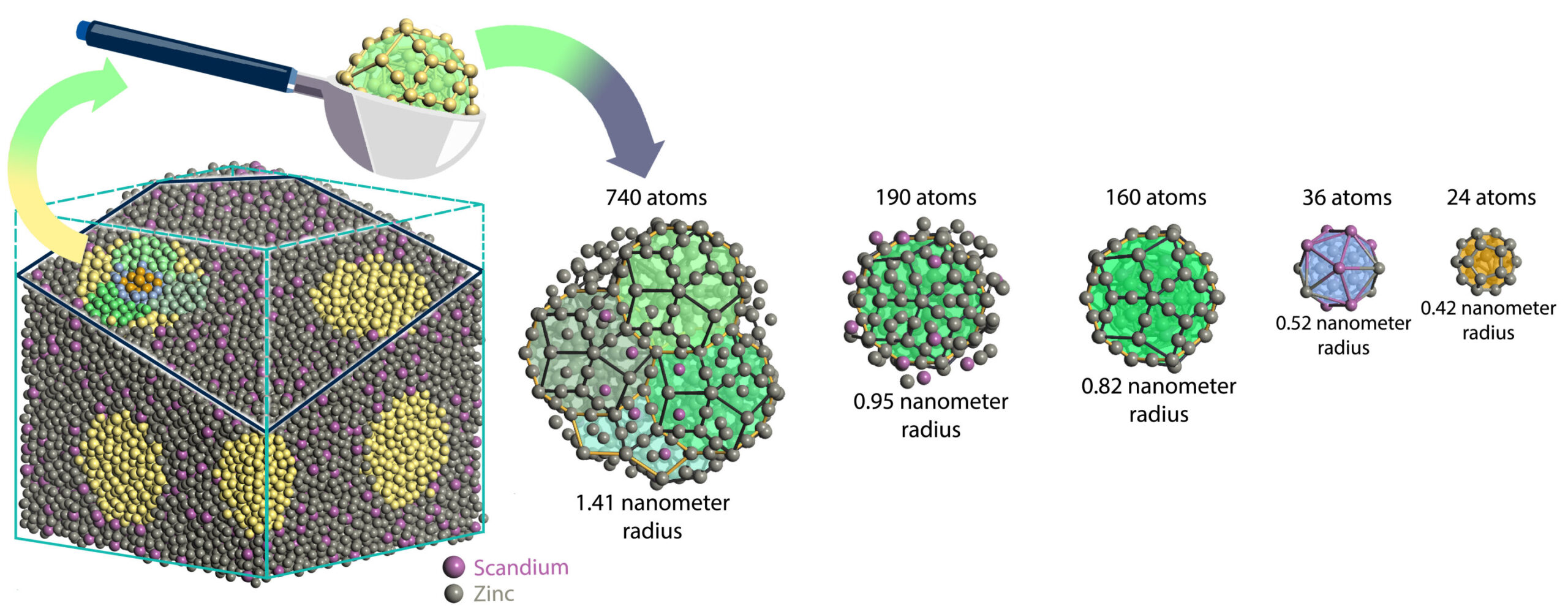
Sun and his team, including doctoral student Woohyeon Baek, found a way around this roadblock by shifting the focus from infinite patterns to finite ones. They didn’t simulate the whole structure. Instead, they studied tiny chunks—nanoparticles—scooped from a larger simulated block of quasicrystal.
A Puzzle of Atoms and Energy
Here’s the trick: the energy of a material tells you a lot about its stability. Atoms naturally want to settle into the lowest energy configuration possible. That’s why crystals form—to minimize energy through ordered bonding. Some materials, like glass, form not because they’re low in energy, but because they’re trapped in a high-entropy state where many disordered arrangements are possible.
Quasicrystals? Scientists couldn’t say.
Using their new approach, the Michigan team calculated the energy of quasicrystal nanoparticles by considering their surface area and volume, then scaling those results up to larger sizes. They did this for two well-known quasicrystals: a scandium-zinc alloy and an ytterbium-cadmium compound.
What they found was astonishing. Both materials are enthalpy-stabilized—meaning they naturally settle into a quasicrystal structure because it’s the lowest energy state. In other words, they aren’t metastable oddities, frozen in place by chance. They’re the real deal—crystals in their own right, just of a new, previously unimagined kind.
“We now have quantum-level evidence that quasicrystals are fundamentally stable structures,” said Baek. “That’s a big deal.”
The Supercomputing Breakthrough
Of course, none of this would have been possible without a serious upgrade in computing power.
Standard algorithms struggled to simulate even modestly sized quasicrystal particles. Doubling the number of atoms made simulations eight times slower. That’s when co-author Vikram Gavini, a U-M professor of mechanical engineering, stepped in with a fix.
“In conventional algorithms, every processor needs to communicate with all the others,” Gavini explained. “Our approach uses localized communication between neighboring processors and leverages GPU acceleration. It’s up to 100 times faster.”
Thanks to this innovation, the team could scale their simulations enough to get precise energy readings and validate the true stability of quasicrystals.
And with this new capability comes an even broader horizon: simulating glass and amorphous materials, modeling crystal defects, and perhaps even probing the materials that could support future quantum computers.
A New Chapter in Material Science
The implications of this work ripple far beyond quasicrystals. If scientists can now model solids that don’t repeat, they’ve broken open an entire category of materials once deemed too complex to understand. That means new alloys, smarter ceramics, stronger composites, and even advances in energy and quantum technology.
Most of all, this research challenges the basic assumptions that have guided material science for decades. For years, the idea of five-fold symmetry was forbidden. Quasicrystals were thought to be accidents. And simulations were limited to materials that followed the rules.
But as this study shows, the universe doesn’t always follow the rules we expect. Sometimes, it surprises us with structures that are neither crystal nor chaos, but something richer, more nuanced—and entirely their own.
Beyond the Impossible
What Daniel Shechtman saw under the microscope in 1984 was more than a new form of matter. It was a lesson in scientific humility. Quasicrystals reminded us that even the most established theories can be upended by a single observation—and that the natural world often holds secrets more elegant and strange than our equations predict.
Now, with quantum simulations confirming what the eye saw decades ago, those secrets are finally being translated into knowledge.
And with each new insight, we move closer to mastering the invisible architecture of atoms—no longer just interpreting the structures nature gives us, but designing our own with precision, creativity, and a deeper understanding of how matter holds itself together.
In a world defined by uncertainty and discovery, quasicrystals are no longer outliers. They are pioneers.
Reference: Baek, W et al. Quasicrystal stability and nucleation kinetics from density functional theory. Nature Physics (2025). DOI: 10.1038/s41567-025-02925-6, www.nature.com/articles/s41567-025-02925-6