It was in 1860 that German physicist Gustav Kirchhoff articulated a law that would go on to shape more than a century of science and technology: that any material’s ability to absorb thermal radiation must equal its ability to emit it—when measured at the same angle and wavelength. This elegant symmetry, known as Kirchhoff’s law of thermal radiation, became a bedrock of physics.
But now, more than 165 years later, a team of engineers at Penn State University has cracked that symmetry wide open—with a force and clarity never seen before.
In research set to be published June 23 in Physical Review Letters and already available on the arXiv preprint server, the team reveals that they have broken Kirchhoff’s law with unprecedented strength. Their experiment didn’t just nudge the boundaries—it smashed through them. And their work, chosen as an Editor’s Suggestion by the prestigious journal, could mark a turning point in how we harvest solar energy, transfer heat, and design the next generation of infrared sensors.
A Law Challenged, a Principle Broken
For decades, scientists believed Kirchhoff’s law was universal: If a surface absorbs a certain amount of electromagnetic radiation at a given wavelength and angle, it must emit the same amount when heated. This reciprocity was like a see-saw in perfect balance.
But recent theoretical work has suggested that under special conditions—such as when a magnetic field is applied—this symmetry could break. A few experiments in recent years hinted at this possibility. They observed subtle mismatches in what certain materials absorbed versus what they emitted. But the difference was always small, the band of radiation narrow, and the technology difficult to scale.
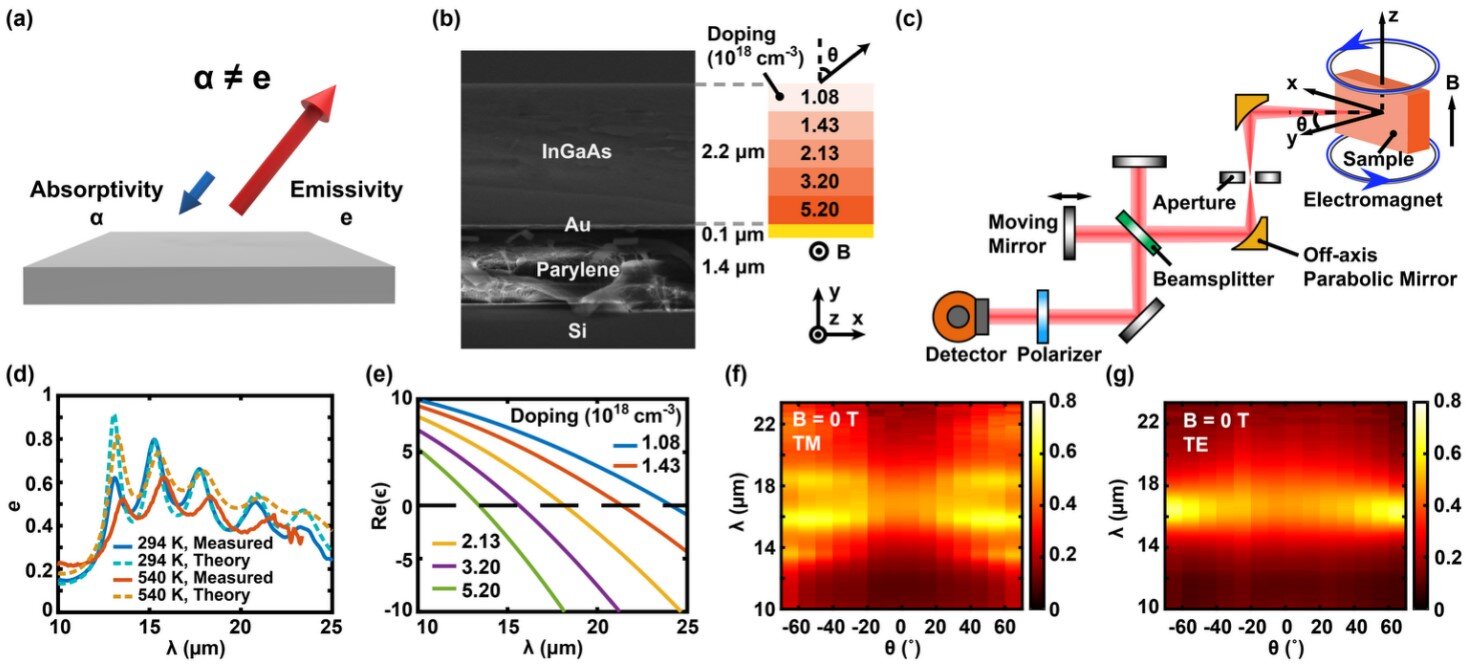
Now, thanks to novel material engineering and a specially designed spectrophotometer, the Penn State team has observed the strongest violation of Kirchhoff’s law ever recorded—with a contrast of 0.43 over a broad wavelength band of 10 micrometers. For context, previous studies achieved contrasts between 0.12 and 0.34, often in much narrower bands.
“In a truly reciprocal system, the contrast between emissivity and absorptivity should be zero,” said Dr. Linxiao Zhu, the study’s senior author and an assistant professor of mechanical engineering at Penn State. “But we’ve built a system where that balance is dramatically broken.”
A Paper-Thin Material With Massive Potential
At the heart of the discovery is a remarkably thin film—just two micrometers thick, less than the width of a human hair. Built from five carefully engineered semiconductor layers, each with slightly different compositions, the material behaves like an orchestra of harmonized absorbers and emitters. Its unique design creates multiple resonance peaks, allowing it to interact with a broad range of infrared wavelengths.
“Our material is not only ultra-thin, but it can also be transferred onto other surfaces,” explained co-first author Alireza Kalantari Dehaghi, a doctoral student in mechanical engineering. “This gives it enormous flexibility for integration into real-world devices.”
In other words, this is not a laboratory curiosity. It’s a functional material that could be applied to solar panels, thermal imaging systems, spacecraft heat shields, and even wearable electronics.
“The ability to control the direction and intensity of thermal radiation opens up entirely new design strategies,” said co-first author Zhenong Zhang. “In solar energy, for example, if your solar cell can avoid sending energy back toward the sun, you’re not just being clever—you’re improving efficiency at a fundamental level.”
Redefining the Rules of Thermal Energy
To measure this groundbreaking phenomenon, the researchers didn’t rely on off-the-shelf tools. They built a custom, angle-resolved magnetic thermal emission spectrophotometer—a complex mouthful of a machine that does something simple but powerful: it measures how much radiation a material emits and absorbs, across a vast range of angles, temperatures, wavelengths, and magnetic fields.
“This system allows us to measure thermal emission in a wavelength-resolved, angle-resolved, and temperature-controlled manner, all while applying a large magnetic field,” Zhu said. “That’s key to seeing such strong nonreciprocity.”
Their setup mimics conditions that might exist in futuristic energy systems—where devices must manage heat and light in dynamic, often extreme environments.
More Than Physics—A Technological Inflection Point
To the average person, breaking a 165-year-old law might sound like a mere academic curiosity. But for engineers and designers, it signals a paradigm shift in how we think about energy.
In traditional solar cells, some energy is inevitably lost, because the device emits light back toward the sun. That’s the reciprocal “give-and-take” Kirchhoff described. But what if that light could be directed elsewhere—toward another solar cell, a power-harvesting mirror, or a sensor? What if we could capture more of the energy that would otherwise be wasted?
Or consider spacecraft that operate in the frigid vacuum of space, exposed to intense heat from the sun. If engineers could tailor how much thermal radiation is emitted in different directions, they could manage temperatures with far greater precision—and without adding weight or complex moving parts.
Even medical diagnostics could benefit. Thermal imaging systems used in hospitals or battlefield triage could be fine-tuned to see more clearly in specific wavelength bands, without being hampered by traditional emission limits.
Looking Ahead: A New Era of Nonreciprocal Design
The Penn State researchers are not done. They plan to explore how other materials behave under similar conditions, and how their custom spectrophotometer can be pushed further. Already, their success shows that the dream of nonreciprocal thermal control is not just possible—it’s practical.
“Strongly breaking Kirchhoff’s law provides a new lens through which we can rethink energy, sensing, and even communication technologies,” Zhu said.
Their breakthrough may very well inspire a new generation of engineers to question what other ‘unbreakable laws’ might simply be waiting for the right materials, the right tools, and the right minds.
Because when you change the rules, you don’t just discover new science. You unlock new ways to build the future.
Reference: Zhenong Zhang et al, Observation of Strong Nonreciprocal Thermal Emission, arXiv (2025). DOI: 10.48550/arxiv.2501.12947