For centuries, chemistry has been bound—quite literally—by distance. Atoms and molecules, the tiny building blocks of everything we see, touch, and breathe, interact only when close enough for their electron clouds to mingle. In nature, this usually means a separation of about the same size as the particles themselves—mere nanometers. Beyond that, their mutual pull weakens into nothingness, leaving them unable to form bonds.
But now, a breakthrough at the Max Planck Institute for the Science of Light (MPL) in Germany has upended this fundamental rule. Using a highly specialized optical device, physicists have managed to “bond” molecules that are much farther apart than nature would normally allow—not by bringing them physically closer, but by bathing them in a specially engineered field of light.
The achievement, published in the Proceedings of the National Academy of Sciences, opens the door to new hybrid states of matter and light, a feat that could one day revolutionize quantum technologies and chemical engineering.
The Fragility of Quantum Conversations
To understand why this is so remarkable, imagine molecules as dancers on a tiny stage. They can only perform together if they’re close enough to clasp hands—close enough for their electrons to overlap and form a stable chemical partnership. This process, known as molecular hybridization, is the basis of bonding in chemistry.
But if the dancers are placed several nanometers apart, the “music” of their electromagnetic signals fades, and they can no longer coordinate their movements. Their interaction simply vanishes.
That’s where the MPL team, led by Professor Vahid Sandoghdar, decided to change the rules. Rather than forcing the molecules closer, they altered the stage itself—reshaping the very fabric of the quantum vacuum surrounding them so that the molecules could “hear” each other again.
The Magic of a Quantum Mirror Box
At the heart of the experiment is a plano-concave microresonator—a device made from two mirrors of extraordinary quality, capable of trapping light between them for unusually long periods. Inside this microcavity, light bounces back and forth in a confined space, its electromagnetic field growing stronger and more coherent with each reflection.
Into this tiny chamber—only a few micrometers across—the researchers inserted a microcrystal of anthracene doped with special dye molecules. These dye molecules acted as the “dancers,” while the trapped light in the resonator acted as both the dance floor and the invisible thread connecting them.
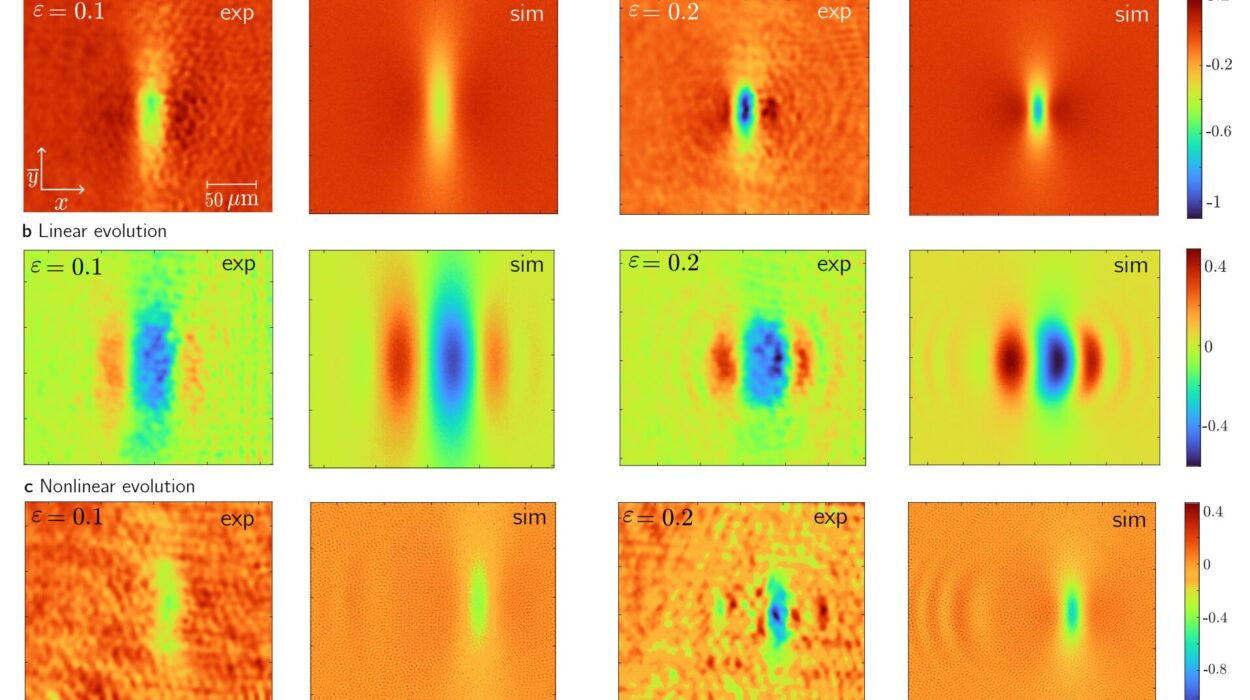
Using high-resolution laser spectroscopy, the team observed how the molecules interacted with the confined light. What they saw went beyond normal chemistry: the molecules’ energy states shifted in ways that revealed entirely new modes of interaction.
Subradiance, Superradiance, and Quantum Harmony
In the altered spectrum, two unusual patterns emerged—subradiant and superradiant states. In subradiant states, the molecules emitted light less strongly than before, as if dampened by the presence of their partner. In superradiant states, they interacted more strongly with the light, amplifying each other’s presence.
These changes meant that the molecules were no longer behaving like isolated entities—they were sharing their quantum states. Even more astonishingly, the team achieved two-photon excitation of two molecules at once. In other words, the molecules could be lifted into an excited state only when two photons arrived together from the resonator. A single photon couldn’t do it, and neither molecule could do it alone—but together, they danced to the tune of paired light particles.
This cooperative behavior is something nature simply doesn’t allow at such distances under normal conditions.
“Gluing” Molecules with Light
Professor Sandoghdar describes the work as “gluing molecules together with light.” While traditional chemistry relies on electrons and overlapping orbitals, this approach relies on engineering the quantum vacuum itself—the invisible background field that permeates empty space. By modifying it within the microresonator, the team created an environment where molecules could form synthetic bonds without being physically close.
“Quantum states are usually very fragile,” Sandoghdar notes, “so coupling multiple molecules together is a significant challenge. Our work is a foundation for developing novel states where particles are connected purely through light.”
Why This Matters for the Future
The implications of this breakthrough stretch far beyond exotic molecular states. In quantum information processing, the ability to precisely control interactions between individual particles is crucial. Systems like the one developed at MPL could allow scientists to design quantum bits (qubits) that communicate in completely new ways, potentially leading to faster, more reliable quantum computers.
It also suggests the possibility of designer chemistry—constructing entirely new types of matter with properties tuned by light fields, not just by the natural constraints of electron overlap.
In essence, this research hints at a future where we can build molecules not only from matter but also from light, unlocking combinations nature never dreamed of.
A Step Into the Unknown
For now, this achievement is a proof-of-principle, a glimpse into what’s possible when physics and chemistry meet in the strange domain of quantum optics. But history has shown that today’s quantum curiosities often become tomorrow’s technologies—from lasers to transistors to MRI scanners.
The MPL team’s success doesn’t just bring molecules together—it brings science closer to a future where the laws of interaction are written not only by nature but also by human imagination.
More information: Jahangir Nobakht et al, Hybridization of molecules via a common photonic mode, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2505161122