Imagine if your body could be taught to clean itself of harmful cells—cancerous growths, rogue immune cells, or other troublemakers—without the need for harsh drugs or invasive treatments. That vision may soon become reality thanks to a breakthrough from researchers at Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS).
In a recent study published in Nature Biomedical Engineering, the team unveiled a remarkable protein-based tool called Crunch—short for Connector for Removal of Unwanted Cell Habitat. This innovation doesn’t act like a traditional drug. Instead, it recruits the body’s own waste-disposal system to quietly and efficiently sweep away problematic cells.
For decades, medical science has searched for ways to target harmful cells without harming healthy ones. Crunch may be the closest we’ve come to a truly elegant solution.
Nature’s Hidden Janitors
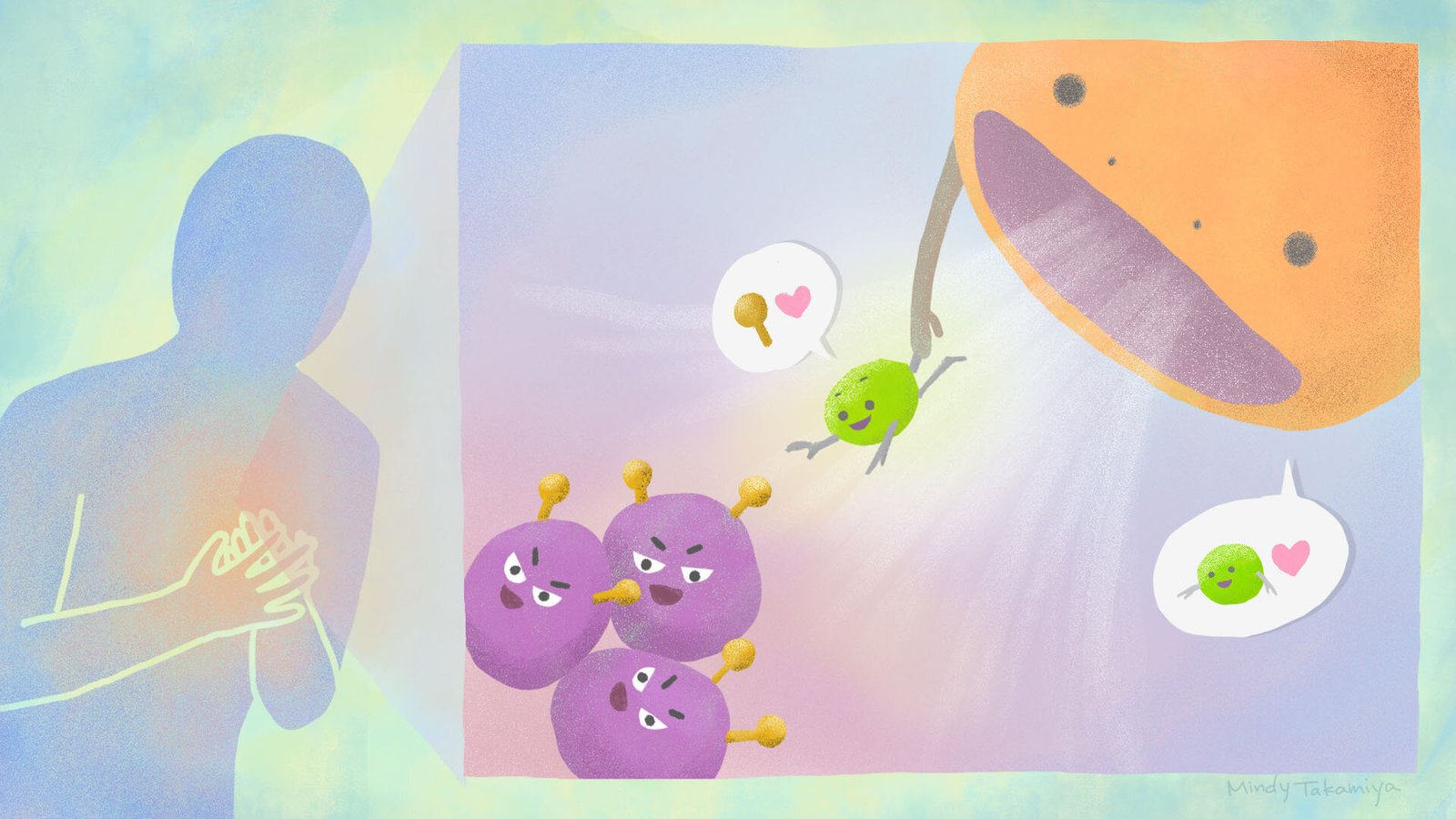
Every second of every day, countless cells in our body reach the end of their natural lifespan. They shut down, send out a quiet signal for help, and wait to be cleared away. That’s when immune cells called phagocytes step in.
Phagocytes are nature’s janitors. They patrol our tissues, scanning for cells that display a molecular “eat me” signal on their surface. Once spotted, the phagocyte engulfs the dying cell in a process known as efferocytosis—from the Latin effere, meaning “to bury.”
This system is so efficient we barely notice it happening. If it didn’t work, our bodies would quickly clog up with dead or malfunctioning cells, leading to disease. What the Kyoto team realized was stunningly simple: What if we could redirect this cleanup crew to remove living cells that were causing harm?
Reprogramming the Cleanup System
The genius of Crunch lies in how it retools this natural system. Normally, a protein called Protein S helps phagocytes recognize dead cells. The researchers, led by Professor Jun Suzuki, asked a daring question: What if we could re-engineer Protein S to recognize living cells that shouldn’t be there?
To do this, they replaced Protein S’s normal “dead cell detector” with a new kind of sensor designed to recognize unique features on harmful cells—like cancer cells or the hyperactive immune cells involved in autoimmune diseases.
When Crunch finds such a target, it doesn’t attack directly. Instead, it acts like a delivery tag, fastening the unwanted cell to a phagocyte. The immune system then does the rest, sweeping the cell away as if it were already marked for disposal.
As first author Yuki Yamato explained, “We took a system the body already trusts and simply rewired it to act on new instructions.”
Precision Without Destruction
One of the most striking things about Crunch is its precision. Unlike chemotherapy, which attacks both healthy and cancerous cells, or immunotherapies that sometimes overstimulate the immune system, Crunch does not force destruction. It gently persuades the body’s janitors to include certain troublemakers in their daily cleaning list.
This makes Crunch both safer and potentially more versatile. Because its targeting sensor can be swapped out and redesigned, it functions as a customizable platform. In principle, a physician could choose the right “sensor” for the condition at hand—whether that’s a tumor, overactive immune cells, or even cells hijacked by infection.
Professor Suzuki put it this way: “It’s an ecosystem for therapeutic tools. Crunch can borrow targeting sensors from antibodies or CAR-T cells, but it works in a simpler and more natural way.”
Proof in Mice: Cancer and Autoimmune Disease
To test their invention, the researchers turned to mouse models. They engineered certain cancer cells with specific surface markers, then deployed Crunch to seek them out. The results were striking: phagocytes efficiently engulfed the cancer cells, and tumor burden was reduced.
They also tested Crunch in a model of lupus, an autoimmune disease where immune cells turn against the body’s own tissues. By programming Crunch to remove these rogue immune cells, they saw a reduction in disease symptoms.
These early results suggest that Crunch could be powerful in contexts where current therapies fall short. Treatments like CAR-T therapy—in which immune cells are removed, genetically altered, and reintroduced—are highly effective but complicated and expensive. Antibody-based therapies, while simpler, sometimes lack long-term durability. Crunch, by contrast, is a protein-based therapy that might one day be given as a simple injection.
Hope for a New Generation of Medicine
The promise of Crunch extends beyond any single disease. It represents a new way of thinking about therapy: instead of fighting the body, we teach it to do what it already does best—maintain balance by cleaning up what doesn’t belong.
Of course, challenges remain. The Kyoto team is now working to improve Crunch’s safety profile, refine its targeting ability, and develop scalable ways to produce it. Clinical applications are still on the horizon, but the principle has been proven: it is possible to redirect the immune system’s housekeeping machinery against harmful cells.
If successful, Crunch could transform how we approach cancer, autoimmune diseases, and beyond. Imagine a future where, instead of harsh treatments, patients receive a tailored protein therapy that quietly reprograms their immune system to restore health.
Science That Feels Like Magic
What makes Crunch so inspiring is not only its clever design but also the elegance of its approach. Rather than inventing something alien to the body, the researchers leaned on the wisdom of nature itself. The immune system has been perfecting the art of cleanup for millions of years. All Crunch does is hand it a new to-do list.
It’s a reminder that science is not always about brute force or radical invention. Sometimes, it’s about listening closely to nature’s own strategies and learning to speak its language.
In that sense, Crunch is more than a therapeutic tool. It’s a glimpse of the medicine of the future—gentle, precise, adaptive, and deeply human.
And perhaps, just perhaps, it’s the beginning of a world where the body itself becomes the doctor, and healing comes from the quiet intelligence of our own cells.
More information: Phagocytic clearance of targeted cells with a synthetic ligand, Nature Biomedical Engineering (2025). DOI: 10.1038/s41551-025-01483-9