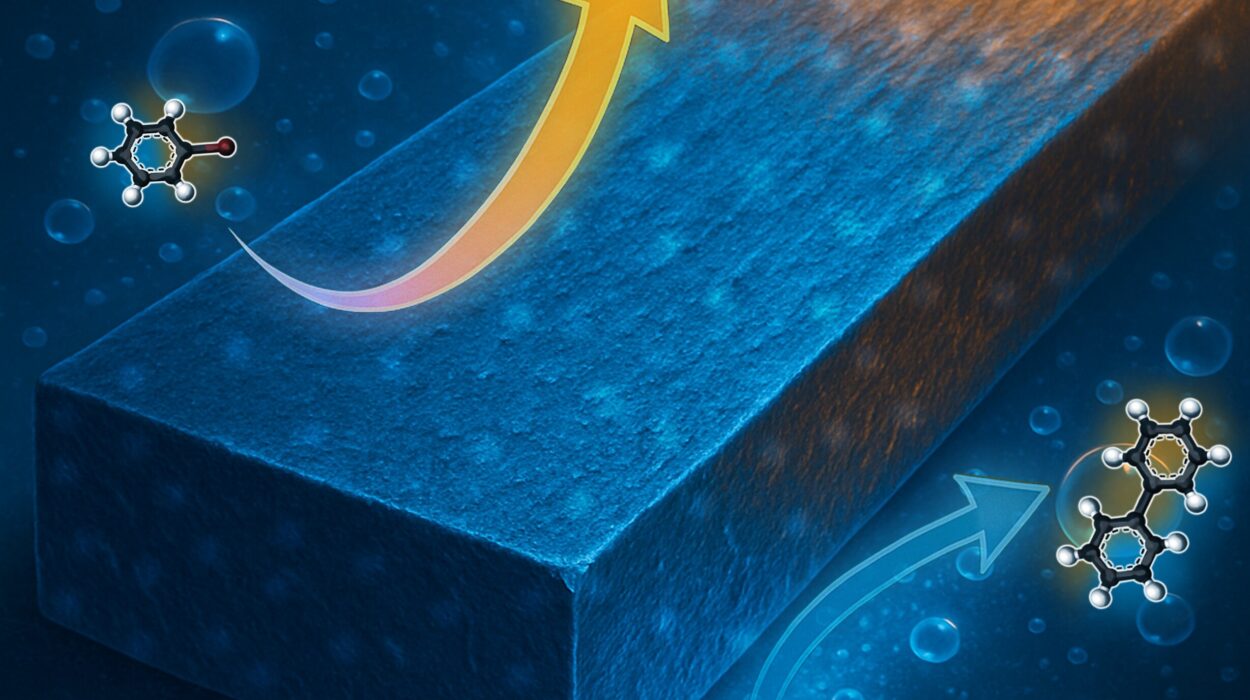
For centuries, chemists have learned to control chemical reactions using familiar tools — raising or lowering temperature, applying pressure, shining light, or adding catalysts. These methods are powerful, but they all depend on direct physical or chemical changes to the system. Now, a team of researchers at the University of Rochester has opened a door to a radically different approach: controlling reactions not by touching the molecules directly, but by altering the quantum “environment” they live in.
In a study published in the Journal of the American Chemical Society, the researchers — led by Frank Huo, the Dean and Laura Marvin Endowed Professor in Physical Chemistry — have developed a theory that explains how a mysterious quantum phenomenon, called vibrational strong coupling (VSC), can speed up or slow down chemical reactions without heat, light, or pressure.
“Our work may provide the first-ever theory that describes the experimentally observed phenomena,” Huo says. “It tells us that the quantum environment alone can influence chemistry in ways we didn’t think were possible.”
Cracking a Scientific Mystery
The mystery began in 2016, when a group of scientists found they could change the speed of a chemical reaction simply by placing the molecules in an unusual setting: a tiny space between two gold mirrors, just millionths of a meter apart. This setup, known as an optical microcavity, doesn’t shine light directly onto the molecules. Instead, it creates a special quantum field in which the natural vibrations of the molecules become “coupled” with the electromagnetic energy inside the cavity.
This is vibrational strong coupling — a strange marriage between light and matter at the quantum level. In some experiments, VSC sped up reactions. In others, it slowed them down. But why it worked at all, and why the effects differed from one case to another, baffled researchers.
Building a Theory from the Quantum Ground Up
For the past five years, Huo and his graduate students, Sebastian Montillo and Wenxiang Ying, have been tackling this puzzle. Using high-powered computer simulations and the principles of quantum mechanics, they’ve built a model that explains the complex dance between molecules and the electromagnetic environment in VSC experiments.
Their theory shows that the quantum environment can change the “energy landscape” of a reaction — subtly shifting the pathways molecules take as they transform from reactants to products. Depending on how strongly the molecular vibrations couple with the light in the cavity, these shifts can make reactions proceed faster or slower.
“This was like solving a challenging jigsaw puzzle,” Huo explains. “All of the puzzling features of VSC finally fit neatly together.”
Why This Matters for the Future of Chemistry
While VSC might sound like an exotic lab trick, it has enormous potential. Being able to control reactions without adding heat or strong light means chemists could make manufacturing processes more energy-efficient and selective. This could lead to greener production of pharmaceuticals, more precise synthesis of advanced materials, and even novel ways to control chemical reactions in biological systems without damaging delicate molecules.
In an era where chemistry is expected to solve problems from climate change to medical innovation, such tools could be game-changers. As Huo puts it, “This new strategy of VSC can selectively slow down or speed up a reaction, offering a paradigm shift in synthetic chemistry.”
The Road Ahead
The next steps will involve applying this theory to real-world reactions, exploring whether VSC can be scaled for industrial use, and discovering how it might combine with traditional chemical controls. While much remains to be tested, the work offers a tantalizing glimpse of a future where chemists can fine-tune reactions simply by adjusting the quantum environment — a future where the invisible fields between mirrors could be as important to chemistry as the heat of a flame or the flash of a laser.
If successful, this could mark the beginning of an entirely new chapter in chemistry — one written not in the language of temperature and pressure, but in the strange, subtle grammar of the quantum world.
More information: Sebastian Montillo Vega et al, Theoretical Insights into the Resonant Suppression Effect in Vibrational Polariton Chemistry, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c03182