Antibiotics have long been the cornerstone of modern medicine, saving millions of lives from infections caused by bacteria like Staphylococcus aureus. However, as the world battles a rising tide of antimicrobial resistance, the simple act of treating these infections is becoming increasingly complex. Among the most notorious offenders in this global crisis is Staphylococcus aureus, particularly its more dangerous cousin, Methicillin-resistant Staphylococcus aureus (MRSA). With resistance to antibiotics on the rise, scientists are scrambling to find alternative treatments, and some are turning to the idea of vaccines. But despite numerous attempts, developing a vaccine against this elusive pathogen has proven challenging—until now.
In a breakthrough study, researchers in China may have discovered a promising new path to creating an effective vaccine against S. aureus infections. By focusing on a specific molecular target, a “surface loop” on an essential bacterial protein, they have bypassed the obstacles that derailed past vaccine efforts, offering a potential game-changer in the fight against drug-resistant S. aureus.
The Challenge of Multidrug-Resistant Staphylococcus aureus
For many, Staphylococcus aureus is a familiar name, as it commonly resides in the human microbiome, particularly on the skin and in the nasal passages. For the majority of people, this bacterium is harmless, even beneficial in some cases, living peacefully alongside other microorganisms in our bodies. However, S. aureus can turn dangerous when it invades deeper tissues, causing infections ranging from mild skin rashes to life-threatening conditions such as pneumonia, endocarditis, and sepsis.
The emergence of multidrug-resistant S. aureus—especially MRSA—has posed a significant public health challenge. MRSA strains are resistant to most antibiotics, making them far more difficult to treat. This has led to increasing hospital-acquired infections and community outbreaks, creating an urgent need for new treatments and, ideally, preventive measures like vaccines. Yet despite extensive research, scientists have found themselves stuck at a crossroads when it comes to developing a vaccine that can effectively protect against these infections.
Why Previous Vaccines Failed
The difficulty in creating a vaccine against S. aureus is, in part, due to the bacterium’s complex relationship with the human body. As mentioned, S. aureus is part of the normal microbiome for many people, particularly in the nasal passages and on the skin. This long-standing coexistence has led to what researchers call a “non-protective immune imprint.” This phenomenon refers to the immune system’s inability to recognize and mount an effective defense against S. aureus because the bacterium is often seen as a harmless resident. The immune system is “trained” to tolerate its presence, which means that any immune response triggered by the bacteria is insufficient to combat more dangerous, pathogenic strains, such as MRSA.
The failure of previous vaccine attempts can be attributed to this very issue. Although some vaccine candidates showed promise in preclinical studies using animal models, they failed when tested in human trials. The immune system’s non-protective imprint, coupled with the bacterium’s ability to evade detection and destruction, made these vaccines ineffective.
A New Approach: Targeting the Surface Loop of MntC
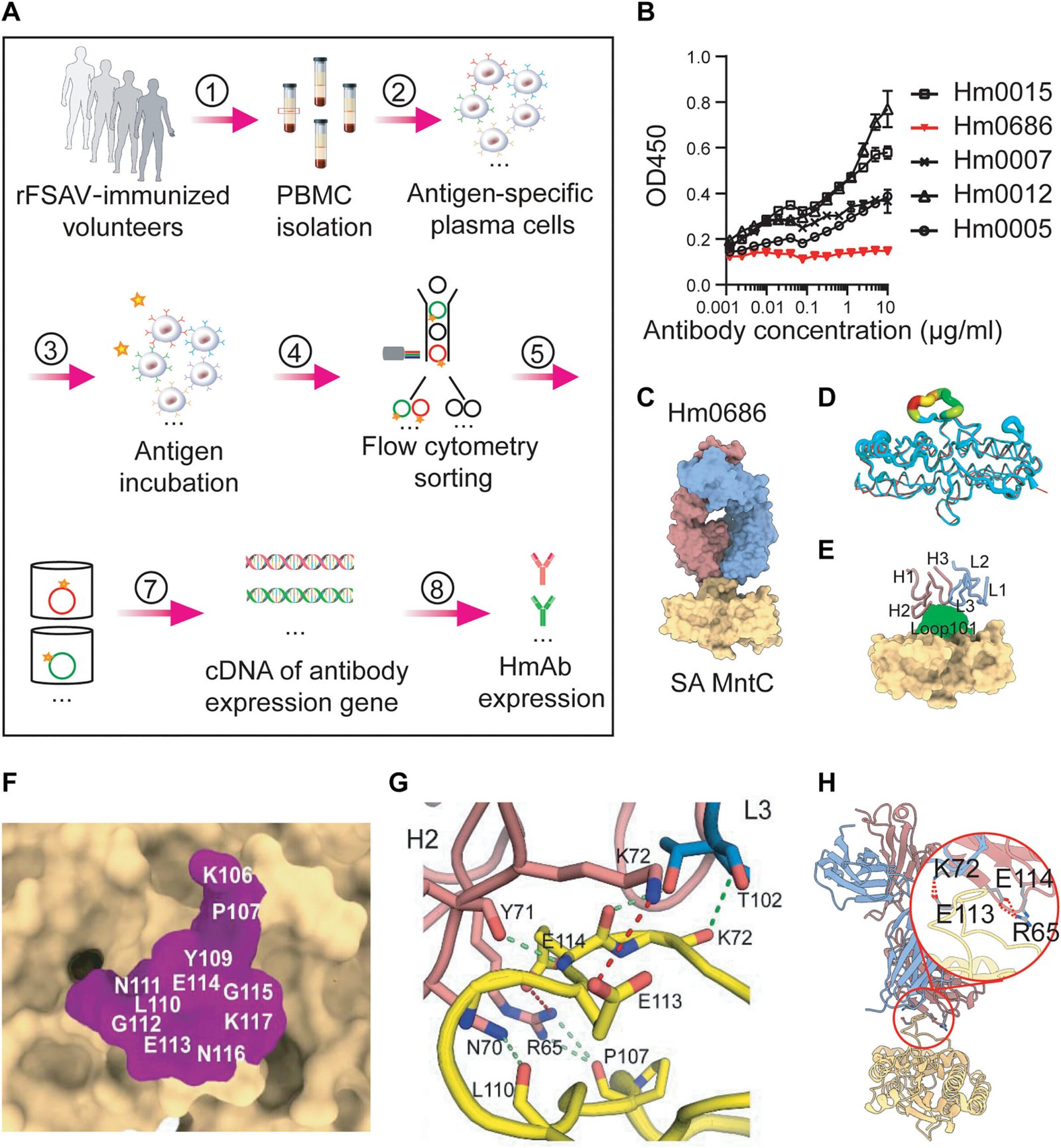
Rather than trying to create a vaccine that targets the entire S. aureus antigen—a strategy that has been stymied by the challenges of immune imprinting—researchers in China have proposed a radically different approach. Instead of aiming for a broad-based immune response, the team has focused their efforts on a very specific and critical region of a key bacterial protein.
In a paper published in Science Translational Medicine, the researchers reveal their innovative strategy: targeting a crucial “surface loop” on an antigen called MntC. This surface loop, known as Loop101, plays an essential role in the survival of S. aureus. Specifically, Loop101 is involved in the bacterium’s ability to absorb manganese, a key metal ion that helps it resist oxidative stress from the host immune system. Without manganese, S. aureus would be unable to survive the harsh conditions inside the human body.
By developing a vaccine that targets Loop101, the team in China has found a way to exploit this survival mechanism. The idea is to stimulate the immune system to produce antibodies specifically against this surface loop. Once these antibodies are in place, they can interfere with the bacterium’s ability to absorb manganese, effectively neutralizing its defenses and making it unable to survive. This approach targets the bacterium’s functional vulnerabilities rather than its broader structure, which is why the researchers are optimistic that it could overcome the obstacles faced by previous vaccine candidates.
Breakthrough Results in Animal Models
The new vaccine, which focuses on this critical epitope (a small but essential piece of the antigen), has already shown promising results in animal models. The researchers used a version of the vaccine that targets Loop101 in S. aureus to immunize mice. When these mice were exposed to S. aureus, the vaccine successfully induced a robust immune response, generating high levels of anti-Loop101 antibodies. These antibodies were able to neutralize the bacteria, preventing infection even in mice that had been previously exposed to S. aureus.
This is a significant achievement, as it suggests that the new vaccine not only prevents new infections but also confers protection against reinfection, a major hurdle in the development of any vaccine.
The Path Forward: Implications for Vaccine Development
The findings of this study could have far-reaching implications for the development of vaccines not only against S. aureus but also against other antibiotic-resistant pathogens. The team’s epitope-based approach could be a more effective and targeted way to develop vaccines against a range of bacteria, from E. coli to Streptococcus pneumoniae, which are similarly resistant to multiple drugs.
The researchers also stress the importance of focusing vaccine development on functional regions of bacterial proteins that are essential for their survival. This is a shift away from the traditional focus on whole antigens, which may not always produce the desired immune response. By targeting specific, functional sites like Loop101, the vaccine is more likely to be effective in generating a protective immune response.
Additionally, Zhang and colleagues propose a new framework for evaluating vaccine efficacy. Instead of merely measuring the concentration of binding antibodies against a whole antigen, they suggest that researchers should focus on testing antibodies specific to critical functional sites, such as Loop101. This more precise measure could offer a clearer understanding of the vaccine’s true immunoprotective potential.
A Glimmer of Hope in the Fight Against Superbugs
The emergence of multidrug-resistant bacteria like MRSA has caused widespread concern, but this new research offers a glimmer of hope. If successful in human trials, this epitope-based vaccine could become a vital tool in the fight against drug-resistant S. aureus infections, saving countless lives in hospitals and communities worldwide.
While there is still much work to be done before this vaccine reaches clinical use, the results so far are promising. By targeting a critical, functional site in the bacterium’s survival mechanism, the vaccine not only offers protection against S. aureus but may also provide a blueprint for developing vaccines against other antibiotic-resistant pathogens.
In a world where the effectiveness of traditional antibiotics is increasingly compromised, this innovative approach represents a bold new frontier in our ongoing battle with superbugs. With continued research and refinement, it may one day become the breakthrough we so desperately need.
More information: Xiaokai Zhang et al, An epitope vaccine derived by analyzing clinical trial samples safeguards hosts with prior exposure to S. aureus against reinfection, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adr7464