Humanity’s race toward a carbon-neutral future hinges on one deceptively simple question: can we make clean hydrogen cheaply, cleanly, and at scale? For years, ammonia (NH₃) has sat on the horizon as a promising hydrogen carrier. It holds abundant H₂ by weight, moves easily through existing infrastructure, and stores well. But the known roadblock has been costly, high-temperature cracking and the expensive purification that follows. Now, a research team at UNIST has pierced that barrier with a deceptively simple and profoundly elegant solution.
Led by Professor Jong-Beom Baek from UNIST’s School of Energy and Chemical Engineering, the team has developed a low-temperature, separation-free system that pulls pure hydrogen out of ammonia while simultaneously producing a valuable industrial material from recycled solar-panel waste. If hydrogen is central to the next energy revolution, this discovery could influence its economics, its waste streams, and its environmental footprint at the same time.
The Old Problem With a Hyped Fuel
Ammonia is widely celebrated in hydrogen-transition strategy documents for good reason: nearly 18% of its weight is hydrogen. It already moves globally in tankers, pipes and storage farms. But extracting hydrogen from it has required temperatures above 400°C—sometimes as high as 600°C—followed by additional cleanup steps. That process burns fuel, consumes money, and erodes the very environmental gains hydrogen is meant to deliver.
Engineers have searched for a way to “crack” ammonia without such heavy heat. None of the proposed catalysts or processes have crossed the needed threshold of cost, simplicity, and purity at the same time. Until now.
A Deceptively Simple Method: Shake, Don’t Burn
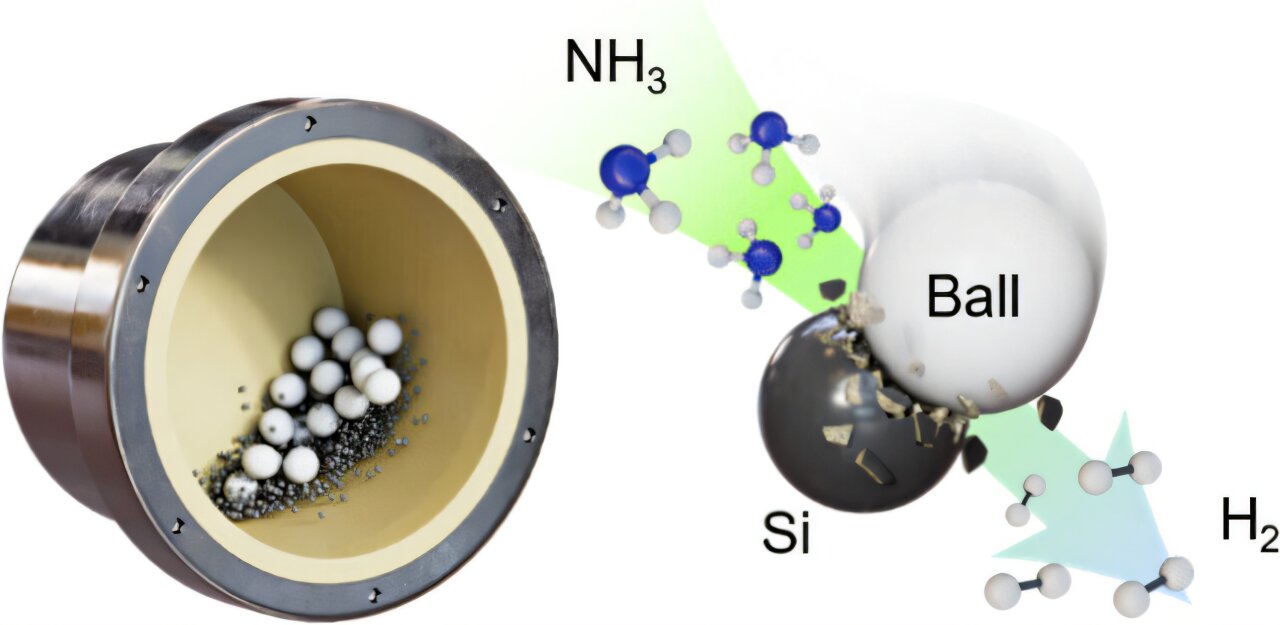
The UNIST team didn’t reach for unattainable alloys or reactors. They turned instead to mechanochemistry—to force generated by motion. In their approach, ammonia gas and finely powdered silicon are placed inside a vessel called a ball mill, which contains small beads made of ceramic or steel. The mill is shaken intensely. Mechanical strikes and friction activate the silicon’s surface, triggering ammonia to decompose at just ~50°C, far below traditional cracking temperatures.
As the molecule comes apart, hydrogen is released as a pure gas, and the nitrogen binds with silicon to form silicon nitride (Si₃N₄) inside the system. Crucially, the nitrogen does not contaminate the hydrogen. That means no downstream purifier, no extra energy penalty, no post-processing complexity.
Two Outputs, Zero Waste: Hydrogen and Silicon Nitride
Results from the experiments were stunning. Ammonia decomposed completely. Pure hydrogen flowed at a rate measurable at 102.5 mmol per hour with confirmed 100% purity. In any industrial hydrogen route, “100% pure” without a downstream separator is more than a metric; it is a structural economic disruption.
Equally transformative is the second output. The nitrogen leftover from the ammonia does not leak—it reacts fully with silicon to become silicon nitride. This is not a waste solid to be buried. Si₃N₄ is a commercially useful, high-value ceramic whose mechanical properties and stability make it desirable for energy storage.
The team fed the produced Si₃N₄ into lithium-ion battery architecture. Cells made with it delivered 391.5 mAh/g and held over 80% of their initial capacity after a thousand cycles at a Coulombic efficiency of 99.9%. Instead of leaving a waste stream behind, the process manufactures a sellable energy-sector co-product.
Turning Yesterday’s Waste Into Tomorrow’s Fuel
Even more striking is the source of the silicon itself. The team tested not only laboratory-grade Si powder but also silicon harvested from retired solar panels. Globally, photovoltaic waste is on track to exceed 80 million tons by 2050. What is now an impending environmental liability could become a hydrogen input stream.
The results were identical: the waste-derived silicon performed as well as commercial silicon. Hydrogen purity remained at 100%, ammonia decomposition was complete, and Si₃N₄ co-production was unaffected. The meaning is clear: energy waste from yesterday can power the energy infrastructure of tomorrow.
When Clean Hydrogen Becomes Profitable Hydrogen
Clean technology is not judged by physics alone; the ledger determines what scales. The UNIST researchers ran the numbers. When the co-product—Si₃N₄ made from recycled silicon—is sold, the economics invert. Instead of costing money to produce hydrogen, the process becomes revenue-positive. The estimate approaches −7.14 USD per kilogram of hydrogen. In plain terms: instead of paying to make hydrogen, a producer could earn money while doing it.
This flips the canonical bottleneck of hydrogen adoption. For two decades, the question has been “Can it get cheap enough?” The UNIST findings suggest there may be routes where hydrogen is not merely inexpensive—it is net-profitable because it is built atop a recycling ecosystem.
The Strategic Consequence
Professor Baek framed the wide-angle view clearly: the technology answers not just one engineering difficulty but an intertwined set—hydrogen separation, cost reduction, and solar-panel waste accumulation. In the long arc of the energy transition, such multi-vector solutions are rare and valuable, because energy systems are not built in clean silos. They intersect in ports, in policies, in grids, in chemistry and in commerce.
This mechanochemical pathway could influence investment decisions far outside academia. It could reshape how ammonia hubs are designed, how solar-waste regulation is written, how hydrogen corridors are priced, and what raw materials are stockpiled.
A Door Opens to a Different Hydrogen Economy
The breakthrough does not invalidate other hydrogen routes—electrolyzers, methane reforming with capture, plasma cracking, photochemical approaches. But it inserts a disruptive option that bypasses major cost penalties attached to separation and heating. It also links two separate sustainability problems—hydrogen delivery and photovoltaic waste—and turns their interaction into an advantage instead of a burden.
If hydrogen is to matter on the scales required for steel, shipping, fertilizers, and long-duration storage, its economic and material flows must be radically simplified. The UNIST work points to a model where cracking, purification, recycling, and value creation are folded into one continuous act of chemistry driven by vibration, not flames.
From Laboratory Insight to Infrastructure Logic
This discovery will still require scaling, techno-economic validation in industrial conditions, durability studies, and policy integration. But conceptually, something important has shifted. It is no longer obvious that ammonia cracking is a thermal problem. It is no longer inevitable that purification is a permanent cost. And it is no longer guaranteed that energy waste is only a liability.
Where traditional processes spent energy to get hydrogen free, this one converts mechanical motion into an economic engine that pays twice: once in hydrogen, once in ceramic value. If taken to industrial scale, it could bend the cost curve of hydrogen downward while bending the waste curve of solar upward into new supply.
At a moment when climate math is unforgiving, breakthroughs like this are more than laboratory news. They are proof that the energy transition can uncover design spaces where constraint becomes opportunity—where one process can cool the planet, feed the grid, and clean up yesterday’s mistakes in the same stroke.
More information: Seung-Hyeon Kim et al, Separation-Free High-Purity Hydrogen Production via the Mechanochemical Ammonia–Silicon Reaction under Mild Conditions, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c10245