Batteries are often seen as symbols of modern technology, powering everything from smartphones to electric vehicles. Yet the idea of storing energy in aqueous systems—where water serves as the electrolyte—has been around for centuries. These aqueous batteries are not new; they have long been valued for their safety and relatively low cost. Unlike many conventional batteries that rely on flammable, organic solvents, aqueous batteries reduce the risk of fire and offer an inherently stable platform.
Still, for all their advantages, aqueous batteries have struggled to gain traction in cutting-edge applications such as large-scale grid storage or next-generation electric vehicles. The main challenge has been compatibility. Many electrode materials, especially those designed for high-performance energy storage, simply do not work well in water-based systems. Some materials lose stability, others degrade too quickly, and many fail to deliver the energy capacity needed to compete with established technologies like lithium-ion batteries.
This longstanding barrier has kept aqueous batteries from stepping fully into the spotlight—until now.
The Challenge of Organic Polymers
Among the materials explored for aqueous systems, organic redox polymers have shown promise. These materials, made of repeating molecular structures, can undergo reversible chemical reactions to store and release charge. They are appealing because they can be made from abundant elements, potentially reducing reliance on rare or toxic metals.
But organic polymers bring their own set of challenges. Hydrophobicity—the natural tendency of certain organic molecules to repel water—has limited their use in aqueous batteries. A material that resists water is not an ideal candidate for a water-based electrolyte. Beyond that, polymers can be difficult to recycle, and they often decompose in ways that make long-term stability problematic. These barriers have kept researchers searching for a way to make organic redox polymers compatible with aqueous environments without sacrificing performance or sustainability.
A Breakthrough from Tohoku University
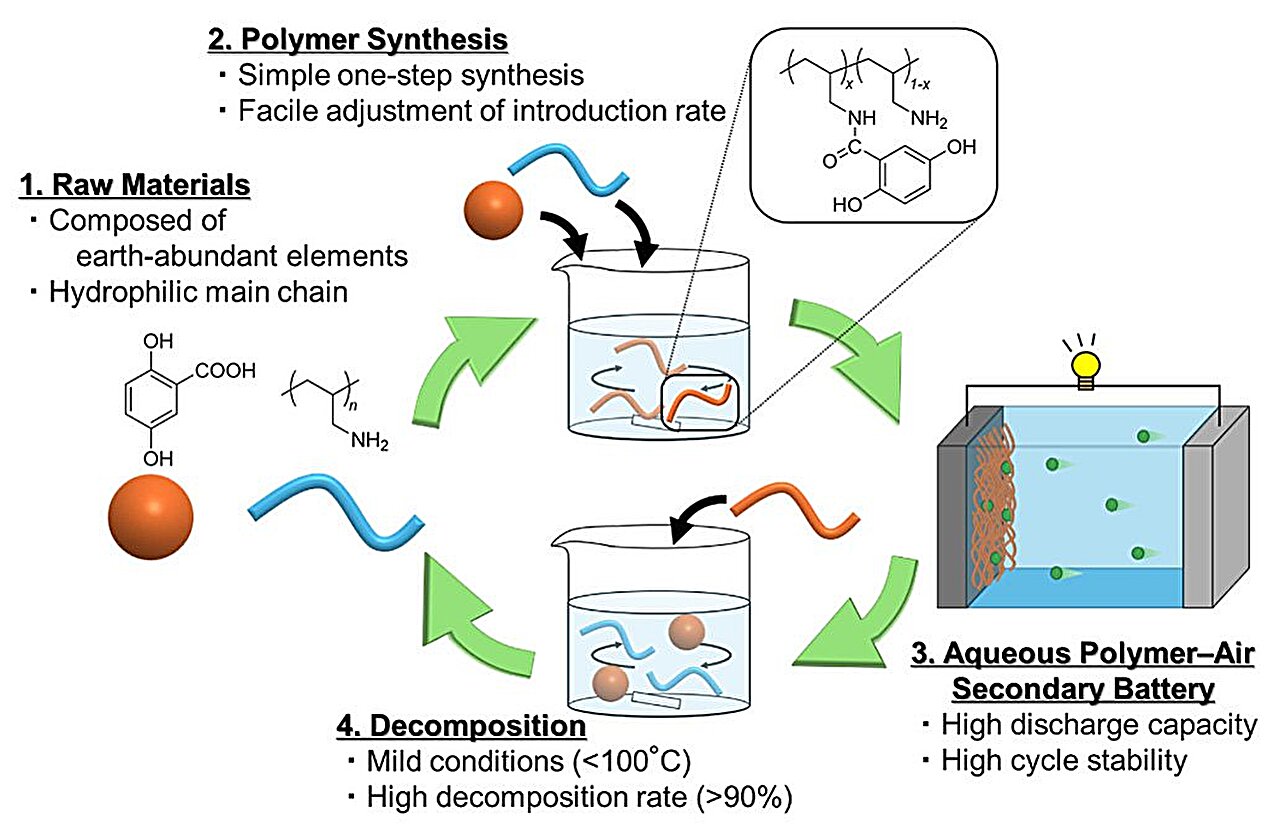
In a recent study published in Polymer Journal, researchers from Tohoku University, working in collaboration with Nitto Boseki Co., Ltd., have taken a significant step toward overcoming these barriers. Their solution lies in clever molecular design—an approach that transforms hydrophobic molecules into water-friendly, high-performing electrode materials.
The team introduced p-dihydroxybenzene, an organic molecule with strong charge storage potential, into a polyamine, which is naturally water-soluble thanks to its positive charge. The process, achieved through a straightforward condensation reaction, produced a polymer that is both hydrophilic—able to mix easily with water—and highly effective as an electrode-active material.
This new polymer works at room temperature, around 25°C, and, remarkably, it can be broken down into its original components under mild conditions at temperatures below 100°C. That recyclability offers not only practical benefits but also environmental ones, pointing to a more sustainable future for battery materials.
Safe, Recyclable, and Sustainable
The breakthrough carries two especially important implications.
First, by using water as the electrolyte, the batteries avoid one of the major risks associated with conventional systems: fire. Lithium-ion batteries, though powerful, are notorious for their flammable solvents, which can catch fire under stress or damage. Aqueous batteries sidestep this hazard, making them safer for large-scale applications such as grid energy storage, where reliability and security are paramount.
Second, the recyclability of the new polymer represents a leap toward sustainability. Made from abundant elements, the material avoids reliance on scarce resources. And because it can be easily decomposed under mild conditions, it does not contribute to the growing problem of plastic waste. Instead of being a burden at the end of its life cycle, the material can return to its original form, ready for reuse.
As Kouki Oka, associate professor at Tohoku University’s Institute of Multidisciplinary Research for Advanced Materials, explained, “By combining high charge storage capacity with recyclability, we can open new directions for sustainable battery research.”
Toward Real-World Applications
While the development marks a major advance, the journey is not over. The researchers emphasize that more work needs to be done, particularly in testing durability and long-term performance. Batteries in real-world applications face countless cycles of charging and discharging, along with exposure to variable conditions. To become a viable competitor to existing technologies, the new polymer-based aqueous batteries will need to prove their resilience as well as their efficiency.
Still, the potential is undeniable. If these materials can deliver on their promise, they could change the energy storage landscape. Imagine a future where the electricity from solar panels or wind turbines is stored safely in large aqueous batteries, without the risk of fire and without the burden of non-recyclable materials. Imagine electric vehicles powered by systems that are not only efficient but also environmentally responsible. This is the horizon the Tohoku University team is pointing toward.
A Future Built on Water
Energy storage is one of the greatest challenges of the modern age. As the world moves toward renewable energy and electrification, the demand for safe, sustainable, and affordable batteries will only grow. Aqueous batteries, long overshadowed by flashier technologies, may finally have their chance to shine.
The work from Tohoku University is more than just a scientific achievement; it is a reminder of how innovation can reshape old ideas. By reimagining what polymers can do, the researchers have breathed new life into a centuries-old concept, transforming aqueous batteries from a niche technology into a contender for the future of energy.
In the end, the story of aqueous batteries is about more than chemistry or engineering. It is about the human drive to take what exists, improve it, and align it with the values of safety, sustainability, and responsibility. It is about building technologies that do not just power our devices but also respect our planet.
The promise of aqueous batteries is a promise of balance: harnessing energy without igniting danger, creating power without creating waste. And with breakthroughs like this, that promise is coming closer to reality.
More information: Kouki Oka et al, Hydroquinone-substituted polyallylamine: redox capability for aqueous polymer–air secondary batteries and recyclability, Polymer Journal (2025). DOI: 10.1038/s41428-025-01085-x