For as long as humans have walked the Earth, we have shared it with invisible adversaries—microscopic enemies that invade our cells, sabotage our organs, and sometimes extinguish life itself. Among the most insidious of these is a single-celled parasite known as Plasmodium. Though invisible to the naked eye, Plasmodium has cast a long and bloody shadow over human history. It is the microscopic killer behind malaria, a disease that has shaped human evolution, toppled empires, and continues to kill hundreds of thousands every year.
The story of Plasmodium is not just a tale of a parasite. It is a story of survival, adaptation, and an evolutionary arms race so intimate that it unfolds within the very red blood cells of its human hosts. Malaria is not merely a disease; it is a relationship—a deadly, intimate entanglement between protist and person.
What Is Plasmodium?
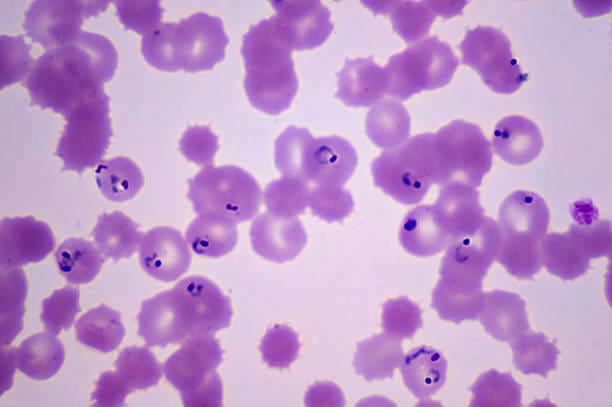
Plasmodium is a genus of protists—unicellular eukaryotic organisms—that belong to the phylum Apicomplexa. These microscopic parasites are distinguished by a specialized structure called an apical complex, which allows them to penetrate host cells. There are over 200 known species of Plasmodium, but only five are known to infect humans: Plasmodium falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi. Among these, P. falciparum is the most dangerous, responsible for the vast majority of severe illness and death.
Unlike bacteria, which are prokaryotes, or viruses, which aren’t truly alive in the conventional sense, Plasmodium is a fully-fledged eukaryote. Its genome is complex, its life cycle bewilderingly intricate, and its survival strategy ruthlessly effective. It is a master of disguise, able to change its surface proteins to avoid immune detection, and a cunning manipulator, capable of altering the behavior of its insect and human hosts to maximize its spread.
The Mosquito Connection
Plasmodium is not a lone killer. It relies on an unlikely accomplice: the mosquito. Specifically, the female mosquito of the genus Anopheles serves as both vector and incubator. When a mosquito bites an infected human, it ingests Plasmodium gametocytes—precursor sexual cells that begin the parasite’s complex life cycle within the insect’s gut. There, they fuse into zygotes, develop into motile ookinetes, and burrow into the mosquito’s midgut wall to form oocysts. Within these oocysts, thousands of sporozoites mature and eventually migrate to the mosquito’s salivary glands.
When the mosquito bites another person, it injects these sporozoites into the bloodstream. And so, in a matter of seconds, the parasite invades a new human host, beginning a destructive journey that will unfold over days or weeks.
This vector-based transmission is what makes malaria both terrifying and intractable. The mosquito does not just act as a vehicle—it acts as a stage for sexual reproduction and diversification, allowing Plasmodium to evolve and adapt at alarming rates. It is in the mosquito that malaria renews its biological assault, generation after generation.
The Life Cycle Inside the Human Body
Once inside the human bloodstream, the sporozoites make their way to the liver within minutes. There, hidden from the immune system, they invade liver cells and multiply rapidly. Each infected cell can release thousands of merozoites, which re-enter the bloodstream and begin invading red blood cells.
This is the stage of the life cycle that causes the symptoms of malaria. Inside the red blood cells, Plasmodium grows and multiplies, digesting hemoglobin and producing toxic waste. Eventually, the infected cell bursts, releasing a new wave of parasites and triggering the host’s immune response—fever, chills, sweating, and pain.
This cycle of red blood cell invasion and destruction repeats every 48 to 72 hours, depending on the Plasmodium species. The synchrony of parasite replication with the release of inflammatory compounds is what causes the characteristic waves of fever and chills that define malaria. The body burns with fever not just because it is infected, but because it is fighting back—raging against an invisible invader that hides within its very cells.
Malaria’s Many Faces
Malaria is not a uniform disease. Its manifestations vary depending on the infecting Plasmodium species, the immune status of the host, and genetic factors such as the presence of sickle-cell trait or other hemoglobinopathies.
Plasmodium falciparum causes the most severe form of malaria. It can lead to cerebral malaria, in which parasites clog the tiny blood vessels of the brain, leading to seizures, coma, and often death. It can also cause severe anemia, respiratory distress, and multi-organ failure. Without treatment, falciparum malaria can be fatal in as little as 24 hours.
P. vivax and P. ovale can lie dormant in the liver for months or even years, reawakening unpredictably to cause recurrent illness. This ability to relapse makes them especially difficult to eradicate.
P. malariae causes a more chronic infection that can last for decades and occasionally leads to nephrotic syndrome, a form of kidney disease.
P. knowlesi, once thought to infect only macaques, has emerged as a zoonotic threat in Southeast Asia. It reproduces rapidly, and misdiagnosis can lead to dangerous delays in treatment.
In all its forms, malaria is a shape-shifter—a disease that refuses to be pinned down by simple definitions or one-size-fits-all treatments.
A Disease That Shaped Civilization
Malaria is as old as humanity. Ancient texts from China, India, and Egypt contain descriptions of intermittent fevers and chills that match its symptoms. Hippocrates wrote about “seasonal fevers” that arose from marshy environments—an early intuition of the mosquito connection.
The disease has shaped human history in profound ways. It has influenced the locations of cities and the outcomes of wars. It helped protect sub-Saharan Africa from European colonization for centuries, as colonizers succumbed to the disease in droves. It played a role in the fall of the Roman Empire and the fate of European imperial expeditions.
Even our genes bear the scars of our long battle with malaria. The sickle-cell mutation, thalassemia, G6PD deficiency, and other inherited blood disorders all offer partial protection against Plasmodium, at a terrible cost. These are evolutionary trade-offs—genetic defenses forged in the crucible of suffering.
The Fight for Control
Modern medicine has made enormous strides in the fight against malaria. Quinine, derived from the bark of the cinchona tree, was the first effective antimalarial. Later came chloroquine, mefloquine, artemisinin, and combination therapies. Mosquito control strategies—bed nets, insecticides, and larvicides—have saved millions of lives.
Yet the battle is far from over. Plasmodium falciparum has developed resistance to nearly every drug thrown at it. Mosquitoes too have evolved resistance to insecticides. The parasite and its vector adapt with a speed that challenges human innovation.
Efforts to create a vaccine have been underway for decades. In 2021, the World Health Organization approved the RTS,S/AS01 vaccine for children in high-transmission areas. It is a historic breakthrough, but only partially effective, and requires multiple doses. Still, it offers hope—a proof that a vaccine is possible.
Gene-drive technologies, which seek to genetically modify mosquitoes to prevent them from carrying Plasmodium, are also under development. But they raise complex ethical and ecological questions. What happens when we begin editing ecosystems at the genetic level? Can we predict the consequences?
The Human Toll
Malaria is not just a disease—it is a humanitarian crisis. It kills more than 600,000 people every year, most of them children under the age of five in sub-Saharan Africa. Every death is a story unfinished—a child who will never grow up, a mother lost in childbirth, a family torn apart.
The disease traps communities in cycles of poverty. It weakens economies, overwhelms healthcare systems, and steals futures. Children who survive severe malaria may suffer long-term cognitive impairments. Adults miss work, families lose income, and nations bear the burden of a preventable plague.
The social cost is immeasurable. Malaria deepens inequality and magnifies injustice. It is a disease of the poor, but not because it must be. With the right tools and political will, it is a disease that could one day be eradicated.
The Mind of the Parasite
To understand Plasmodium is to marvel at its diabolical ingenuity. It is not simply a killer—it is a strategist. It times its reproductive cycle to maximize its transmission. It changes its surface antigens to evade immune responses. It even manipulates the behavior of its mosquito hosts, making them more likely to bite humans when they are most infectious.
Recent research has shown that Plasmodium may alter the scent of infected humans to make them more attractive to mosquitoes, increasing the chance of transmission. It may influence the feeding behavior of mosquitoes to encourage multiple bites.
This is not intelligence in the way we usually define it. But it is a form of evolutionary cunning—a natural selection honed over millions of years to produce a parasite so effective that it has survived everything we’ve thrown at it.
Hope on the Horizon
Despite the immense challenge, there is hope. Global malaria deaths have declined significantly since the early 2000s, thanks to coordinated public health efforts, better diagnostics, new treatments, and international funding. The WHO’s Global Technical Strategy for Malaria aims to reduce malaria case incidence and mortality rates by 90% by 2030.
Innovative tools like insecticide-treated bed nets, rapid diagnostic tests, mobile health technologies, and community-based interventions are saving lives daily. Scientific collaboration, political commitment, and grassroots activism are converging in ways that once seemed impossible.
But malaria is relentless. Setbacks—such as drug resistance, funding gaps, and political instability—can reverse hard-won gains. The fight requires not only scientific progress, but sustained compassion and global solidarity.
The Parasite and Us
What does it mean to live in a world where a single-celled organism can defeat the might of civilizations? Where a child dies every minute from a parasite that fits inside a red blood cell?
It means we are not just battling biology—we are battling complacency. We are battling indifference. Malaria is not inevitable. It is a disease of inequality, of neglect, of failure to act.
Plasmodium is deadly, but it is also vulnerable. It depends on a chain of events that we can interrupt. We can close the door on the mosquito. We can cut the parasite’s life short with medicines. We can vaccinate, educate, prevent, and heal.
In this ancient war between protist and person, we are not powerless. But we must decide what kind of world we want to live in. One where children are born under mosquito nets, or one where they die without ever seeing their fifth birthday. One where science is used not just to understand the world, but to change it.
Conclusion: A Future Without Malaria?
Plasmodium is one of the most formidable pathogens humanity has ever faced. It is ancient, adaptable, and intricately entwined with our biology and our history. Malaria has killed more people than any other infectious disease in human history, and it continues to do so today.
Yet even this killer is not beyond our reach. The story of malaria is not just a story of suffering—it is a story of resilience, ingenuity, and hope. From quinine to vaccines, from mosquito nets to gene drives, humanity has never stopped fighting.
The future is unwritten. We stand at the crossroads of possibility. Will we let this ancient enemy continue its rampage, or will we bring an end to the longest war in human history?
The answer, as always, lies with us.