Pain is a universal experience, shared across species, shaping behavior and survival instincts. It alerts us to danger, compels us to withdraw from harm, and fuels our understanding of the human condition. But pain isn’t monolithic—it comes in many flavors: sharp, burning, stabbing, throbbing. A hot stove and a pinched finger trigger very different sensations, yet how does our nervous system tell the difference?
For decades, neuroscience has sought to decode the biological language of pain. While much attention has focused on the brain’s role as the ultimate interpreter of suffering, new research is shining light on a lesser-understood player in this sensory drama: the spinal cord. A recent groundbreaking study by researchers at the Karolinska Institute, Uppsala University, and other European institutes reveals a sophisticated spinal sorting system that distinguishes between heat-related and mechanical pain in mice. Published in Nature Neuroscience, their work uncovers previously hidden complexity in how pain is encoded and regulated at the very gateway to the brain.
A New Frontier in Pain Research
Pain begins at the periphery—with receptors in our skin, joints, and organs that detect damage or the threat of damage. These receptors then send electrical signals along nerves to the spinal cord, which acts as a communication hub, routing pain messages upward to the brain.
But the spinal cord isn’t just a relay station. It’s a dynamic processing center capable of modifying, amplifying, or even blocking pain signals before they ever reach the brain. Despite its central role in the early stages of pain processing, the spinal cord’s ability to differentiate types of pain—like heat from a burn versus the sharp jab of a needle—has remained largely mysterious.
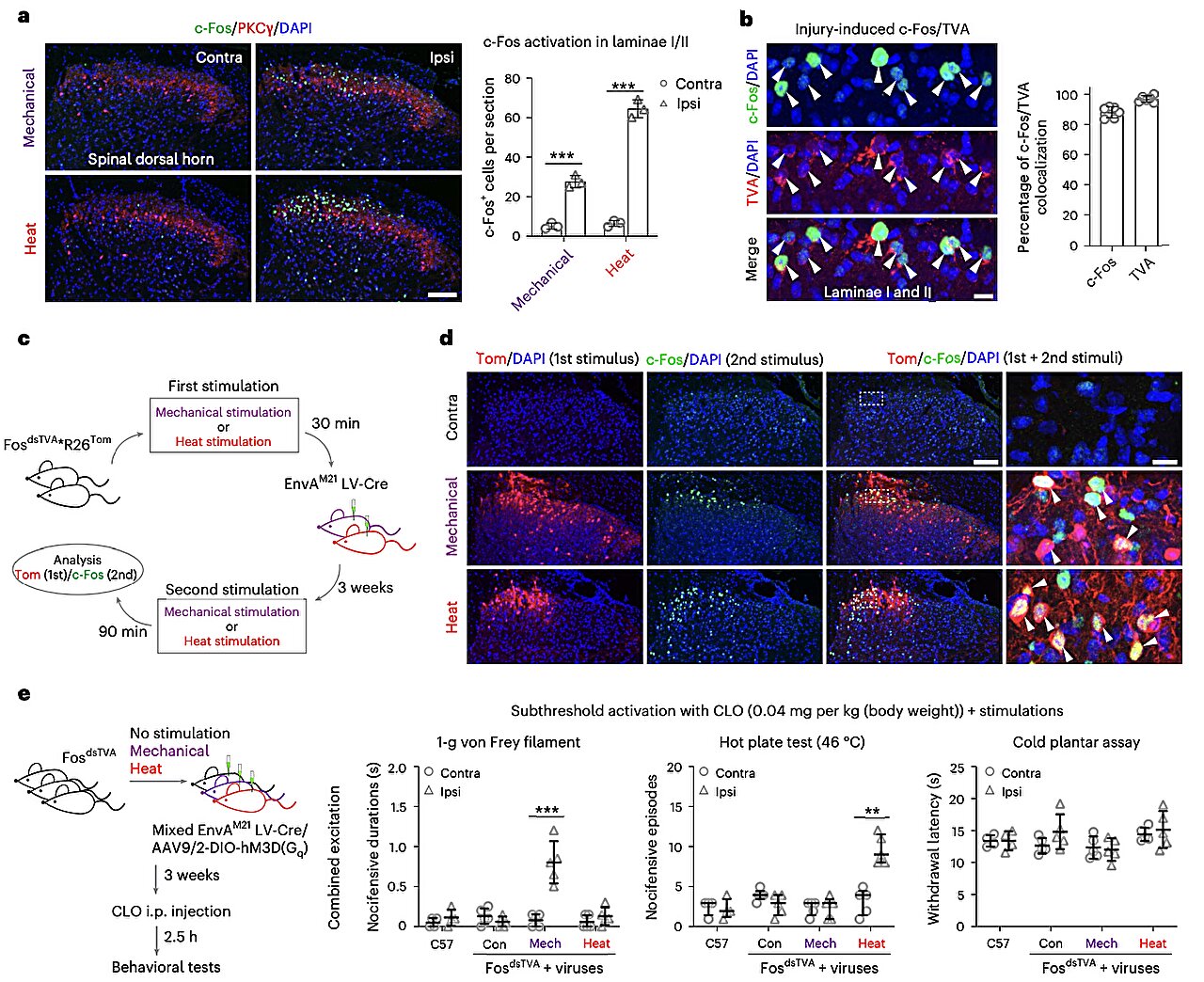
To probe this enigma, researchers turned to advanced genetic tools and behavioral analysis in mice. Using a suite of high-precision techniques—including genetic capturing, activity manipulation, and single-cell RNA sequencing—they traced the activity of individual spinal neurons as the animals experienced different forms of pain.
Heat vs. Hurt: The Discovery of Pain-Specific Neural Circuits
What they discovered was astonishing: the spinal cord contains distinct neural ensembles—networks of neurons—that are specialized to encode specific types of pain. In this case, heat-induced pain and mechanically induced pain (such as pressure or sharp objects) activated entirely separate sets of neurons.
By selectively reactivating or silencing these neuron groups, the researchers were able to essentially “turn on” or “turn off” the sensation of a specific type of pain. When certain ensembles were artificially reactivated, mice displayed classic pain behaviors—paw licking, shaking, and lifting—suggesting they were experiencing discomfort. Conversely, when these neural circuits were silenced, the animals no longer responded to painful stimuli, even when exposed to high heat or physical pressure.
This discovery represents a fundamental shift in our understanding of pain. Rather than being processed by a generalized system, different pain types are managed by distinct, hardwired neural populations right at the spinal level. This organization offers a potential explanation for why certain pain medications work better for some types of pain than others—and why chronic pain conditions can be so difficult to treat.
The Power Players: Galanin-Positive Inhibitory Neurons
Among the most intriguing findings was the role of a special class of inhibitory neurons, known as Gal+ neurons, named for their expression of the neuropeptide galanin. These neurons act as modulators or “gatekeepers,” dampening pain signals before they escalate up the spinal cord.
Gal+ neurons were found to be polymodal—they interact with multiple types of sensory input—and they form direct, monosynaptic connections with A-fiber sensory neurons. This gives them a privileged position in the pain hierarchy. When activated, Gal+ neurons effectively “gate” the transmission of pain, reducing the overall intensity of the sensation. When these neurons were silenced or reduced, the spinal cord’s ability to distinguish between pain types became disrupted.
Even more compelling was the observation that nerve injury, such as that caused by trauma or neuropathy, triggered inflammation driven by microglial cells—the immune cells of the nervous system. This inflammation altered the balance of neural ensembles, decreasing the activity of Gal+ inhibitory neurons and increasing excitatory signals. The result? Heightened pain sensitivity, known as hyperalgesia, often seen in chronic pain disorders.
However, when researchers reactivated Gal+ neurons, they were able to reverse this hypersensitivity. This opens an exciting therapeutic avenue: targeting Gal+ neurons may offer a way to selectively modulate pain without relying on opioids or general anesthetics.
A Precision Map of Pain
The use of single-cell RNA sequencing provided an unprecedented view into the molecular identity of each neuron involved in pain processing. By decoding which genes were active in each cell, researchers built a precise molecular map of the spinal cord’s pain circuits. This molecular fingerprinting could pave the way for future drugs designed to target specific neuron types, minimizing side effects and maximizing relief.
The discovery of modality-specific spinal ensembles adds a powerful new layer to the already complex architecture of pain. It confirms what many have long suspected: pain is not merely a single signal carried by nerves but a nuanced, multi-dimensional experience sculpted by numerous specialized systems.
Implications for Human Health and Chronic Pain
While this study was conducted in mice, the principles it uncovered are likely to hold true in humans. The spinal cord is highly conserved across mammalian species, and previous pain research in mice has reliably translated into human clinical insights.
Chronic pain affects over 20% of the global population, with conditions ranging from arthritis and fibromyalgia to sciatica and phantom limb pain. Many of these conditions involve abnormal signaling in the spinal cord, making this research incredibly relevant for future treatments.
Imagine a new generation of painkillers that don’t simply dull sensation but selectively silence the exact neurons responsible for the specific type of pain you’re experiencing. Or gene therapies that restore inhibitory control in damaged pain circuits, returning the nervous system to its natural balance. These aren’t science fiction—they’re potential realities inspired by discoveries like those of the Karolinska and Uppsala teams.
Beyond Pain: A New Window into Sensory Processing
This study also underscores the spinal cord’s broader role in sensory perception. The concept of neural ensembles could extend beyond pain to include temperature, touch, itch, or even proprioception (the sense of body position). It suggests that the spinal cord may be a far more complex and intelligent processor of sensory information than previously recognized.
As we unravel these circuits, we gain not only tools for relieving suffering but also profound insights into how our bodies interface with the world. Pain, once considered a brute force of biology, is emerging as a finely tuned symphony of neural signals—one that our spinal cord helps compose with remarkable precision.
A Closing Thought
The work by Zhang, Kupari, and their colleagues doesn’t just expand our understanding of pain—it reframes it. It invites us to think of the spinal cord not as a passive conduit, but as a neural gatekeeper, capable of sorting, filtering, and shaping our sensory experience before it ever reaches consciousness.
As we move forward, integrating these findings into clinical practice, the potential to redefine how we diagnose and treat pain is enormous. From neuropathic disorders to inflammatory pain syndromes, from post-surgical discomfort to debilitating chronic pain, the answers may well lie not in the brain—but in the intricate, intelligent circuits of the spinal cord.
Pain, it turns out, is not just what we feel. It’s how we’re wired to interpret our world—and that wiring just got a lot more fascinating.
Reference: Ming-Dong Zhang et al, Neural ensembles that encode nocifensive mechanical and heat pain in mouse spinal cord, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-01921-6.