For years, medicine has fought viruses with a straightforward logic: if a patient doesn’t have enough protective antibodies, supply them from the outside. But a new clinical trial points toward a more elegant future — one in which the body is instructed to manufacture its own specialized defenses on demand. Instead of shipping fragile antibodies around the world and infusing them into patients, researchers have shown that a tiny piece of DNA can enter muscle tissue, deliver genetic instructions, and prompt cells to build the antibodies internally. The study, published in Nature Medicine, marks a rare moment when theory, technology, and need converge into a new chapter for immunotherapy.
Why We Need Another Tool Beyond Vaccines and Traditional Antibodies
The global rollout of mRNA vaccines changed the course of the pandemic, but not everyone responds to vaccination with the same strength. Immunocompromised individuals — transplant recipients, certain cancer patients, the elderly — may never reach adequate immunity through vaccination alone. For these groups, monoclonal antibody drugs offered a critical safety net: lab-made proteins designed to copy the body’s own immune tools and neutralize the virus directly.
But monoclonal antibodies come with weaknesses that limit their reach. They are biologically delicate, must be chilled from lab to bedside, cost enormous resources to manufacture, and lose effectiveness over time. Protection fades as the protein breaks down or as the virus evolves away from its target. These constraints have pushed researchers to search for a method that is longer-lasting, cheaper, and easier to distribute globally — even in places without reliable refrigeration or medical infrastructure.
Encoding Immunity Instead of Shipping It
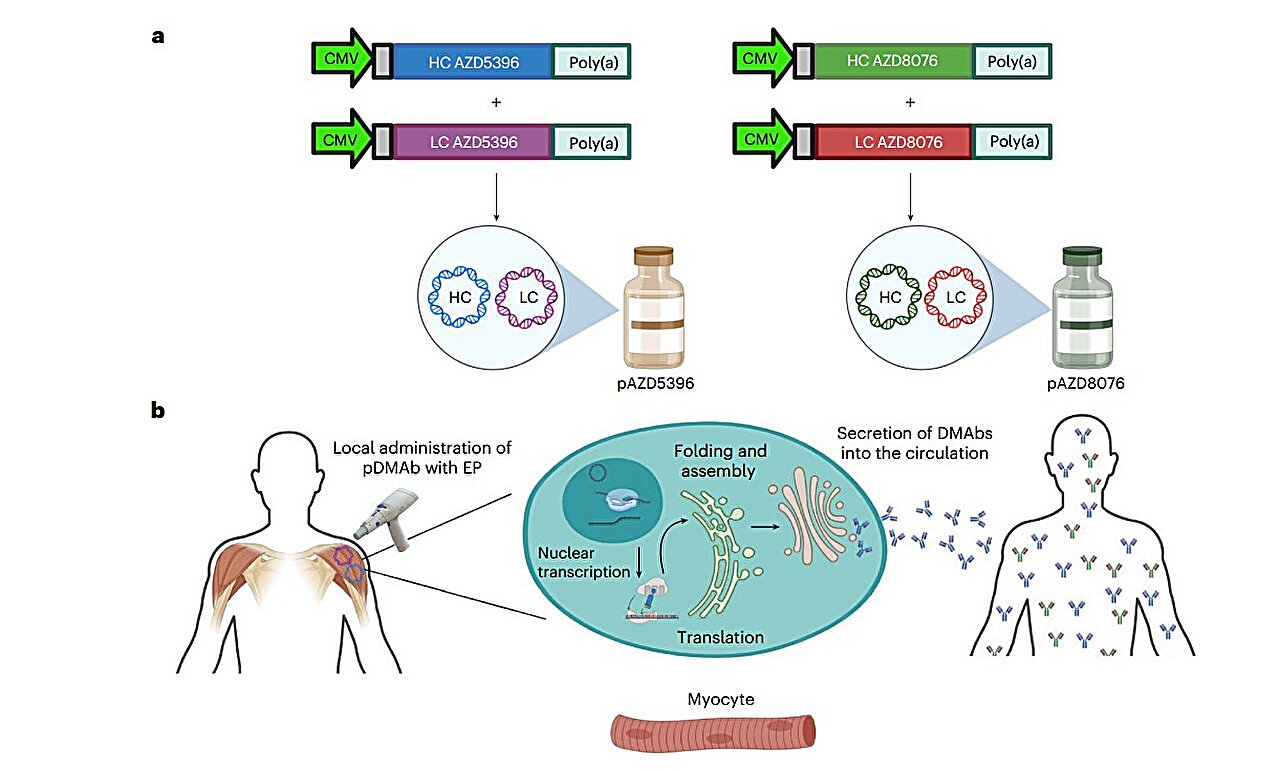
Enter DNA-encoded monoclonal antibodies (DMAbs). Instead of delivering the antibody itself, the DMAb approach delivers blueprints — a small ring of synthetic plasmid DNA encoding the antibodies of interest. Once the DNA enters muscle cells, those cells begin producing the antibodies internally, just as though the body had made them naturally. This idea merges the reliability of monoclonals with the self-renewing efficiency of genetic instruction.
In the Phase I trial, researchers injected DMAbs encoding two SARS-CoV-2-neutralizing antibodies into 44 healthy adults aged 18 to 60. A short electrical pulse after injection encouraged the DNA to slip into cells — a technique known as electroporation. Unlike intravenous antibody therapy, this is a simple intramuscular shot.
The goal was not to test whether the treatment could prevent infection — that will come later — but to answer two foundational questions: is this safe, and does it behave in the body as intended?
Safety, Stability, and the Surprising Duration of Protection
After 72 weeks of monitoring, the results were clear. The platform was well tolerated. Side effects were overwhelmingly mild and short-lived — mainly temporary soreness or redness at the injection site. There were no serious product-related events. Perhaps more importantly, participants did not generate immune reactions against the DMAb itself; the body did not treat the DNA instructions as an invader to be destroyed.
The most striking outcome was durability. Antibodies generated from the DMAb instructions remained active for the entire 72-week observation period — vastly outlasting traditional monoclonal infusions, which decline over weeks or months. This longevity could mean fewer treatments, greater convenience, lower cost, and a better shield for those at highest risk.
Why DNA-Based Antibodies Matter Beyond COVID-19
If the promise holds, DMAbs could rewrite how medicine manages infectious disease. Because the DNA contains instructions rather than fragile protein, it can be shipped without cold-chain storage and produced at scale with relatively low cost. That advantage alone makes it attractive for global health settings where refrigeration and transport are major obstacles.
There is also a strategic advantage: the same platform can be edited and repurposed quickly for new viral strains or emerging pathogens. Instead of rebuilding an antibody therapy from scratch each time a virus mutates, scientists could adjust the code and send a new version into production with minimal delay.
Beyond infectious diseases, DNA-encoded antibodies could be explored for conditions where monoclonals already play a role, such as autoimmune disease, cancer immunotherapy, and chronic inflammatory disorders. The same logic applies: deliver instructions, let the body be the factory.
A Quiet Turning Point in Therapeutic Design
The trial was not meant to solve COVID-19 in one stroke. Its achievement is more fundamental: proof that a human body can safely be taught to manufacture neutralizing antibodies from code alone — without cold storage, without constant re-dosing, without fear of rapid decline. This is a conceptual shift away from a medicine of external supply and toward a medicine of internal instruction.
Not every breakthrough comes with drama. Some arrive as quiet proof-of-concepts that expand the boundaries of what is possible. DMAb technology is one such moment — a reminder that the future of medicine may depend less on what we ship into the body and more on what we convince the body to do for itself.
More information: Pablo Tebas et al, Safety and pharmacokinetics of SARS-CoV-2 DNA-encoded monoclonal antibodies in healthy adults: a phase 1 trial, Nature Medicine (2025). DOI: 10.1038/s41591-025-03969-0