Somewhere deep inside a laboratory, far removed from the buzz of everyday life, a scientist lowers a silvery metallic coil into a chamber colder than outer space. A cloud of liquid nitrogen swirls around it like ghostly breath, chilling it below a temperature so low it barely belongs on Earth. And then—something magical happens. Resistance vanishes. Electric current, once slowed by microscopic collisions and heat, flows endlessly, without losing energy.
This is the strange and wondrous world of superconductors.
A hundred years ago, no one believed such a thing was possible. How could electricity, which always faced resistance in even the purest metals, suddenly glide with absolute freedom? Yet here it was, observed and measured. The laws of physics hadn’t been broken—they had simply revealed a deeper layer, hidden in the frigid quiet of extreme cold. What began as a curious anomaly has evolved into one of the most fascinating frontiers of modern science.
The Discovery That Defied Expectations
The story of superconductivity begins in 1911 with a Dutch physicist named Heike Kamerlingh Onnes. At the time, Onnes was a pioneer in the study of low temperatures. He had already made history by liquefying helium—a gas so resistant to compression that it became a symbol of scientific patience. Using this liquid helium, Onnes began cooling materials to temperatures never reached before.
He chose mercury as his test subject, expecting its electrical resistance to decrease gradually as it got colder. But what he observed was entirely unexpected. At 4.2 Kelvin—just a few degrees above absolute zero—mercury’s resistance didn’t just decrease. It disappeared entirely.
Not reduced. Not minimized. Gone.
This was the first observation of superconductivity. Onnes had discovered a new state of matter, though even he couldn’t fully explain it. His work earned him the Nobel Prize in 1913, but more importantly, it opened a door to a world where the ordinary rules of electricity no longer applied.
The Physics of Perfection
To understand superconductors, we must first understand what usually happens when electricity flows. In a regular conductor—like copper—electrons move through a sea of atoms. But their journey is far from smooth. They constantly collide with those atoms, losing energy in the form of heat. That’s why wires get warm. That’s why motors waste power. That’s why resistance exists.
Superconductors are the complete opposite. Below a certain critical temperature, the material undergoes a phase transition. The electrons stop behaving like independent particles and instead pair up into what are known as Cooper pairs. These pairs act in a synchronized way, forming a quantum wave that moves through the material without scattering.
It’s like the difference between pushing your way through a crowded room and joining a choreographed dance where everyone moves in perfect unison. Suddenly, there’s no more bumping, no more energy loss. The current flows with zero resistance—and it would, in theory, keep flowing forever.
This is not just theoretical. In laboratories, superconducting currents have been observed to persist for years without decay. It’s the closest thing we have to perpetual motion, though governed entirely by the known laws of quantum mechanics.
More Than Just Zero Resistance
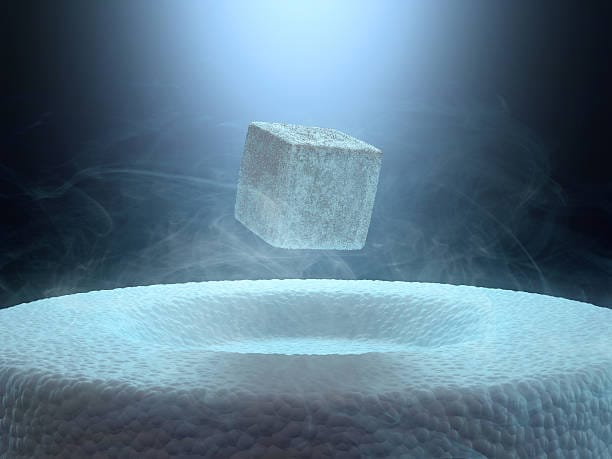
As astonishing as zero resistance is, superconductors have another superpower: they expel magnetic fields. This effect, known as the Meissner effect, was discovered in 1933 by Walther Meissner and Robert Ochsenfeld. When a material becomes superconducting, it pushes out all magnetic fields from its interior. This property gives rise to one of the most visually spectacular demonstrations in physics: quantum levitation.
Place a small magnet above a supercooled superconducting disk, and the magnet will float. Not hover. Float—suspended in mid-air, perfectly stable, as if frozen in time. This phenomenon happens because the superconductor forms tiny electric currents that create magnetic fields exactly opposing the external one. It’s a delicate and perfect balance.
In essence, superconductors not only eliminate resistance—they manipulate magnetism in ways that seem to defy gravity. These twin properties—zero resistance and magnetic expulsion—make superconductors unlike any other known materials.
From Curiosity to Technology
For decades after their discovery, superconductors remained scientific curiosities. The need for extremely cold temperatures—near absolute zero—made them impractical for widespread use. Cooling with liquid helium is expensive, complex, and inefficient for most applications. But things began to change dramatically in the 1980s.
In 1986, two researchers, Karl Müller and Johannes Bednorz, discovered a new type of superconductor made of ceramic materials that operated at a much higher temperature—around 35 Kelvin. Still freezing, but significantly warmer than previous superconductors. Within a year, other researchers had pushed the record above 90 Kelvin, making it possible to use liquid nitrogen as a coolant.
This was a turning point.
Liquid nitrogen is cheap and widely available. Suddenly, superconductors were no longer confined to the lab. They began to creep into technology—quietly, but powerfully.
Today, superconductors are used in MRI machines, particle accelerators, and quantum computers. They are being tested in power grids, maglev trains, and next-generation batteries. Every new discovery pushes us closer to a world where superconducting technologies are part of everyday life.
The Quantum Puzzle
Despite all our progress, the fundamental question remains: why do superconductors work?
The original theory, known as BCS theory, was developed in 1957 by John Bardeen, Leon Cooper, and Robert Schrieffer. It explained how low-temperature superconductors form Cooper pairs through vibrations in the atomic lattice—like tiny quantum dances that let electrons move without resistance.
But BCS theory can’t fully explain the new, high-temperature superconductors discovered in the 1980s and beyond. These materials—mostly ceramics with complex crystal structures—don’t behave like traditional metals. Their electrons interact more strongly. Their properties defy easy classification. Despite decades of research, we still don’t fully understand how they become superconducting.
This is one of the biggest unsolved problems in physics. Cracking it could unlock even higher-temperature superconductors—perhaps even those that work at room temperature. And that would change everything.
A World Transformed
Imagine a world where electricity flows across continents without loss. Where your phone charges in seconds. Where trains float frictionlessly above tracks, gliding silently at 600 kilometers per hour. Where quantum computers solve problems in minutes that would take classical computers millennia. This is not fantasy—it is the world that superconductors could build.
Energy loss in power lines accounts for massive inefficiencies in modern grids. Superconducting cables could eliminate that waste. Hospitals could be equipped with compact MRI scanners. Electric motors—currently limited by resistance—could be lighter, faster, and more efficient.
Even in space exploration, superconductors could play a role, enabling magnetic propulsion systems and more efficient power generation.
The barrier is temperature. Even the best high-temperature superconductors still require cooling. But researchers are racing toward the holy grail: a room-temperature superconductor. If we find one that works under practical conditions, it would be as revolutionary as the discovery of electricity itself.
The Race for Room-Temperature Superconductivity
In recent years, headlines have occasionally exploded with claims of room-temperature superconductors. Some of these claims are true—in part. In 2020, scientists reported a material that becomes superconducting at 15°C (59°F)—technically room temperature—but only under pressures more than two million times that of Earth’s atmosphere. Not exactly practical.
These materials, typically made from hydrogen-rich compounds like carbonaceous sulfur hydride, are opening new paths. But for real-world use, we need superconductors that work at room temperature and normal pressure.
Still, the breakthroughs are coming faster. Advances in material science, nanotechnology, and quantum modeling are helping us design compounds that were once unthinkable. Machine learning algorithms are now being used to predict which crystal structures might be superconducting. What once took decades of trial and error can now be simulated in weeks.
We are not there yet. But the destination is closer than it has ever been.
Challenges and Hope
Despite the promise, superconductors are not a silver bullet. They are brittle. Many are difficult to manufacture. Their critical temperatures are still far below the boiling point of water. Scaling up production, ensuring stability, and integrating them into existing technologies all remain significant hurdles.
But every new discovery adds another stone to the path forward.
One reason for hope is the sheer diversity of fields that benefit from superconductivity. From energy to medicine, transportation to quantum computing, the incentives for progress are massive. The global race includes national labs, private companies, and university research teams across continents.
This isn’t just a scientific quest—it’s an economic and environmental one. A world built on superconductors is a world of greater efficiency, lower emissions, and fewer technological limits. That world may be difficult to reach—but it is not unreachable.
The Beauty of the Impossible
In the end, superconductors are more than just a technological marvel. They are a reminder that the universe still holds mysteries we are only beginning to understand. They whisper of a reality where electrons move in perfect harmony, where resistance ceases, and where the impossible becomes routine.
They challenge our assumptions about what materials can do. They invite us to imagine beyond the constraints of heat, friction, and waste. They show us that the coldest places on Earth can ignite the hottest ideas.
As scientists and engineers continue to unravel their secrets, one thing is clear: superconductors have not just changed physics—they are shaping the future of the world we live in.
And in that future, resistance—literal and metaphorical—may no longer hold us back.