Huntington’s disease is a neurodegenerative disorder that leads to the gradual deterioration of movement, mood, and cognitive abilities. This genetic condition affects people worldwide and tends to progress slowly over many years, ultimately causing severe neurological impairment and death. The disease is caused by mutations in the HTT gene, which encodes a protein called huntingtin. Specifically, the mutation involves an abnormal expansion of repeating DNA sequences—specifically, CAG (cytosine-adenine-guanine) trinucleotide repeats—that causes the huntingtin protein to malfunction.
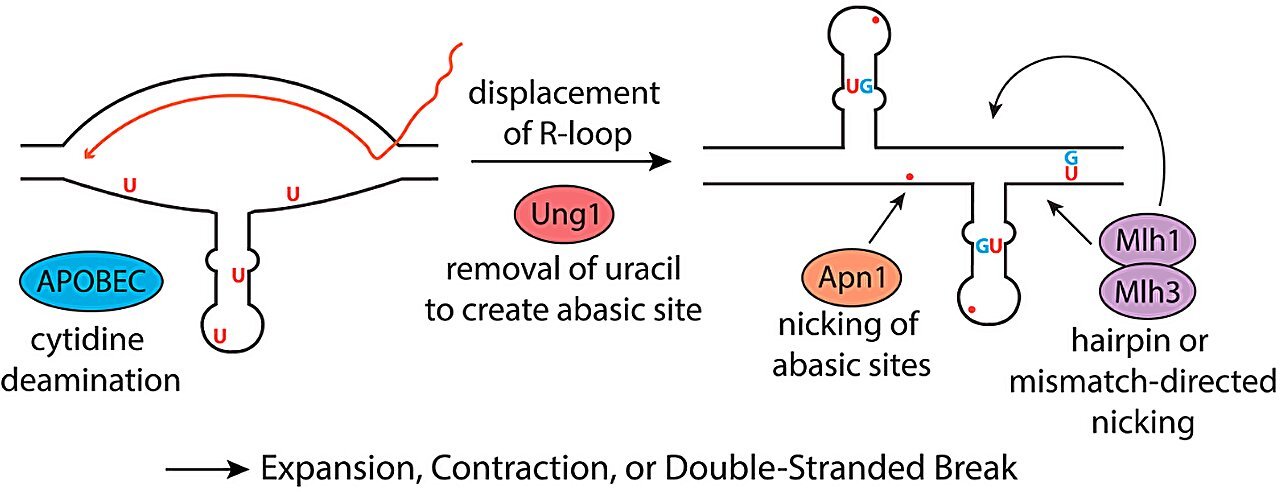
While previous research has illuminated some of the core biological mechanisms leading to Huntington’s disease, understanding the molecular process driving the expansion of these repeats has been challenging. In a groundbreaking study, Catherine Freudenreich, a biology professor, and her research team provide new insights into how the DNA repeats in the Huntington’s gene can be broken and expanded, contributing to the disease’s development. These discoveries point to a surprising culprits: proteins in the immune system designed to defend against viruses, known as APOBECs (Apolipoprotein B mRNA editing catalytic proteins).
The basic understanding of Huntington’s disease revolves around a sequence of CAG repeats within the HTT gene. Normally, the gene may contain up to 35 repeats of CAG, but in individuals with Huntington’s disease, this sequence becomes abnormally lengthened—often extending to 40–120 repetitions. This lengthening creates kinks and distortions in the DNA structure, which in turn increases the likelihood of DNA breaks during replication. Repair mechanisms within the cells fail to fix the breaks correctly, which leads to further expansions of the repeats and contributes to the loss of proper protein functions, ultimately causing nerve cell death.
Freudenreich’s team’s work points to a specific set of proteins—the APOBECs—that may play a key role in initiating and perpetuating this process. Under normal circumstances, APOBECs are part of the body’s innate immune response, primarily tasked with targeting viruses for destruction. APOBECs work by editing viral genomes, often deaminating cytosine nucleotides in single-stranded viral DNA. This leads to mutations that render the viral genes nonfunctional, offering an initial defense against viral infections before the adaptive immune system produces more specific antibodies and T-cells.
However, the team found that in Huntington’s disease, the APOBECs may begin to mistakenly target the body’s own genetic material. Under certain conditions, human DNA can fold into single-stranded loops, particularly in parts of the HTT gene containing the CAG/CTG repeat sequences. The APOBECs then attack this DNA by deaminating the cytosines in these regions, further destabilizing the genetic structure. This provokes an erroneous DNA repair process, whereby the faulty repair enzymes insert extra CAG/CTG repeats, leading to the repeat expansion characteristic of Huntington’s disease.
As part of their research, Freudenreich and her team investigated the distinct roles of various APOBEC proteins to understand which ones were most influential in causing this repeat expansion. There are 11 known types of APOBEC proteins in humans, and the researchers used yeast cells to examine how each type might contribute to the process. They found that APOBEC3A was particularly potent in inducing the expansion of CAG/CTG repeats. APOBEC3B, though less influential, also played a role. These findings are important because the frequency and manner in which these proteins act within cells could influence the severity and progression of Huntington’s disease.
Further supporting these findings, Freudenreich collaborated with Steve Roberts, an associate professor at the University of Vermont. Roberts examined publicly available protein expression data from brain tissue samples of deceased Huntington’s disease patients. This analysis revealed unusually high levels of the APOBEC3A protein in the brains of these patients. Remarkably, these levels were even higher than those seen in breast cancer cells, where APOBECs are already known to contribute to disease progression. These findings indicate that APOBEC3A is present in the exact location—within the brain—where it could act as a trigger for repeat expansion and may contribute to the pathological features of Huntington’s disease.
The implications of this discovery go beyond simply understanding how repeat expansions occur. These findings raise potential therapeutic opportunities. The discovery that APOBEC enzymes play such a central role in the repeat expansion offers a promising direction for therapeutic strategies. For example, inhibiting the action of APOBECs could be a way to prevent or slow the expansion of CAG repeats, thus halting the progression of Huntington’s disease. If specific inhibitors could be developed that target APOBEC activity without disrupting normal immune responses, these inhibitors might be able to prevent or mitigate the neurological damage associated with the disease.
Interestingly, Freudenreich’s findings also raise important questions about the role of viral infections in triggering Huntington’s disease or influencing its progression. APOBEC proteins, known for their viral-defense functions, may become overactive due to viral infections or inflammatory conditions, potentially promoting DNA damage that exacerbates Huntington’s disease. This suggests that an interaction between immune responses to viral infections and the genetic predisposition to Huntington’s disease might trigger or accelerate disease onset. Whether certain viral infections play a significant role in Huntington’s disease, in combination with APOBEC proteins, is an area ripe for future exploration.
Reference: Rebecca E. Brown et al, APOBEC3A deaminates CTG hairpin loops to promote fragility and instability of expanded CAG/CTG repeats, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2408179122