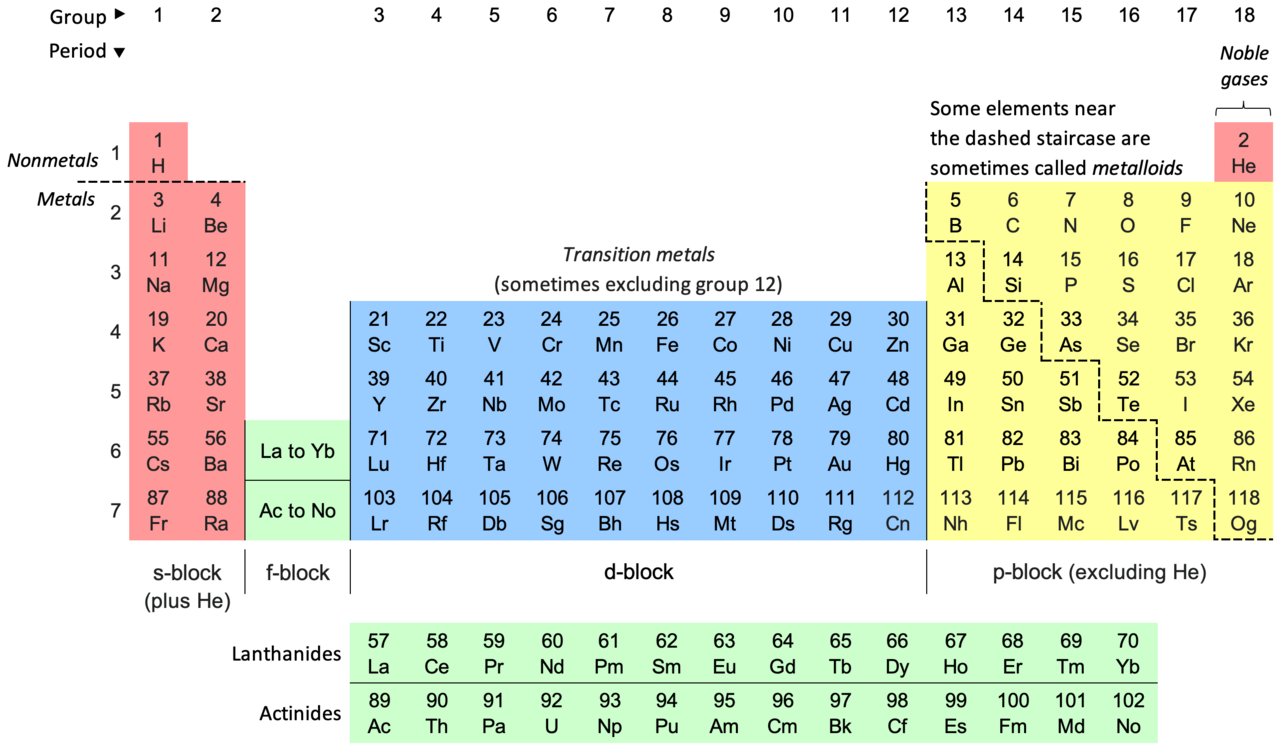
The periodic table may look calm and orderly at first glance, a neat grid of symbols and numbers printed in textbooks and hung on classroom walls. But beneath that tidy surface lies drama, volatility, and raw power. Some elements are quiet and cooperative, content to sit inertly in the background. Others, however, are chemical firebrands. They hiss, burn, shatter bonds, release torrents of energy, and sometimes explode with terrifying enthusiasm. These elements have personalities—unpredictable, aggressive, and unforgettable.
An “explosive personality” in chemistry doesn’t always mean literal explosions, though many of these elements certainly do that. It can mean extreme reactivity, violent energy release, unstable atomic structures, or the ability to transform environments in moments. These elements remind us that matter is not passive. At the atomic level, the universe is restless.
Here are ten elements on the periodic table with the most explosive personalities, each one a character in chemistry’s most dramatic stories.
1. Hydrogen – The Element That Built and Breaks the Universe
Hydrogen looks innocent. It’s the simplest element, made of just one proton and one electron. Yet it may be the most explosively influential substance in existence. Hydrogen fuels the stars, powers nuclear fusion, and has the capacity to unleash unimaginable energy.
On Earth, hydrogen’s explosiveness is more familiar and far more dangerous. When mixed with oxygen in the right proportions, hydrogen becomes violently flammable. A single spark can trigger an explosion powerful enough to tear through steel and concrete. This is not because hydrogen is heavy or complex, but because it is desperate to bond. Hydrogen atoms release enormous energy when they combine with oxygen to form water, snapping into stability with explosive enthusiasm.
Historically, hydrogen’s volatility has left scars. The destruction of hydrogen-filled airships demonstrated just how unforgiving this element can be. In modern science, hydrogen is treated with deep respect. It promises clean energy in the future, yet it also reminds us that harnessing power at the atomic level always comes with risk.
Hydrogen’s explosive personality is cosmic in scale. It creates stars, destroys structures, and sits at the foundation of chemistry itself.
2. Oxygen – The Fire That Makes Fire Possible
Oxygen is essential for life, but it is also one of the most dangerously reactive elements humans encounter daily. Fire, as we experience it, cannot exist without oxygen. It is the silent partner in every flame, the unseen force that turns fuel into destruction.
Oxygen itself does not explode easily, but it makes explosions far more violent. In oxygen-rich environments, materials that normally burn slowly can ignite instantly. Metals can burn like paper. Grease can detonate. A tiny spark becomes catastrophic.
This explosive personality comes from oxygen’s strong desire to steal electrons. It is one of the most electronegative elements on the periodic table, meaning it aggressively pulls electrons toward itself. This property drives combustion, corrosion, and countless chemical reactions.
Even within the human body, oxygen walks a dangerous line. It sustains life, yet excessive oxygen can damage cells through oxidative stress. Oxygen is a paradox: gentle breath and raging inferno, healer and destroyer, all wrapped into one deceptively common gas.
3. Sodium – The Metal That Explodes in Water
Sodium is a soft, silvery metal that can be cut with a knife. It looks harmless, even fragile. But drop sodium into water, and the illusion shatters. The metal skitters across the surface, hisses violently, bursts into flames, and sometimes explodes with enough force to shatter glass.
The reason lies in sodium’s electron configuration. Sodium has a single electron it desperately wants to lose. Water provides the perfect opportunity. When sodium reacts with water, it releases hydrogen gas and heat. The heat ignites the hydrogen, and the reaction accelerates faster than it can be controlled.
In nature, sodium is never found alone. Its explosive personality ensures it is always locked into compounds, most famously sodium chloride—table salt. Bound to chlorine, sodium becomes stable, edible, and essential to life. Unbound, it is chaos waiting to happen.
Sodium is a lesson in chemical context. The same element that keeps your nerves firing and muscles contracting can become violently destructive when freed from its constraints.
4. Potassium – Sodium’s Even Wilder Sibling
If sodium is dangerous, potassium is downright reckless. Potassium belongs to the same family of alkali metals, but it is even more reactive. When potassium meets water, the reaction is faster, hotter, and more explosive. Flames leap higher. Shockwaves are stronger. Sometimes the metal detonates instantly.
Potassium’s explosive personality comes from its larger atomic size. Its outer electron is held more loosely, making it easier to lose and more eager to react. This makes potassium invaluable in biological systems, where its controlled movement across cell membranes regulates heartbeats and nerve signals.
But outside the body, potassium is unforgiving. Exposure to moisture, air, or mishandling can lead to fires and explosions. Laboratories store potassium under oil, isolating it from any chance of accidental reaction.
Potassium embodies chemistry’s duality: absolutely essential for life, absolutely intolerant of carelessness.
5. Fluorine – The Most Aggressive Element Known
Fluorine is not just reactive; it is the most reactive element on the entire periodic table. It does not merely participate in reactions—it dominates them. Fluorine attacks glass, ignites organic matter, and reacts violently with nearly every other element, sometimes explosively.
This aggression stems from fluorine’s extreme electronegativity. It has a ravenous hunger for electrons and will tear them from other atoms with astonishing force. Even substances that resist most chemical attacks can fall apart in fluorine’s presence.
Handling fluorine is so dangerous that early researchers were severely injured or killed trying to isolate it. The element is toxic, corrosive, and unforgiving. Yet when tamed, fluorine becomes incredibly useful. It strengthens plastics, protects teeth, and stabilizes pharmaceuticals.
Fluorine’s explosive personality is that of a conqueror. Left unchecked, it destroys. Controlled, it reshapes chemistry itself.
6. Nitrogen – Calm on the Surface, Explosive at Heart
Nitrogen makes up most of Earth’s atmosphere, quietly surrounding us with apparent indifference. It seems inert, harmless, even boring. But this calm exterior hides immense explosive potential.
Nitrogen atoms bond to each other with extraordinary strength. Breaking those bonds releases enormous energy. This stored energy is unleashed in nitrogen-rich explosives such as TNT, nitroglycerin, and ammonium nitrate. In these compounds, nitrogen’s desire to return to its stable form drives devastating explosions.
Lightning, too, demonstrates nitrogen’s hidden power. The energy of a lightning bolt breaks nitrogen molecules apart, allowing them to react and form new compounds. Even life itself depends on nitrogen’s controlled transformation, as plants and bacteria convert it into usable forms.
Nitrogen’s personality is deceptive. It waits patiently, storing energy, until conditions are right. Then it releases that energy with terrifying force.
7. Phosphorus – The Element That Burns on Contact with Air
Phosphorus is one of the most dramatic elements known. In one of its forms, white phosphorus ignites spontaneously when exposed to oxygen. No spark is required. Simply meeting air is enough to set it ablaze.
This explosive behavior comes from phosphorus’s unstable molecular structure. White phosphorus stores energy in strained bonds that are desperate to rearrange themselves into a more stable form. When oxygen is present, the release is immediate and violent.
Phosphorus has played dark roles in human history. It has been used in incendiary weapons and caused horrific injuries. At the same time, phosphorus is essential for life. It forms the backbone of DNA, fuels cellular energy, and builds bones and teeth.
Few elements capture chemistry’s moral tension like phosphorus. It is both a cornerstone of biology and a weapon of destruction, depending entirely on how it is handled.
8. Chlorine – The Green Gas with a Violent Temper
Chlorine is a greenish-yellow gas with a sharp, choking odor. It is highly reactive, corrosive, and toxic. Chlorine does not explode easily on its own, but it reacts violently with many substances, releasing heat, fire, and poisonous byproducts.
Chlorine’s explosive personality emerges when it encounters metals, hydrogen, or organic compounds. These reactions can be rapid and uncontrollable. Chlorine gas was infamously used as a chemical weapon, demonstrating its ability to damage tissue and disrupt biological systems.
Yet chlorine is also indispensable. It disinfects drinking water, keeps swimming pools safe, and helps produce countless materials. Bound with sodium, it becomes harmless table salt.
Chlorine teaches a recurring lesson of chemistry: danger and usefulness are often separated by a single chemical bond.
9. Uranium – The Element That Rewrites Reality
Uranium’s explosive personality operates on a scale beyond chemistry. It is a nuclear force, capable of releasing energy millions of times greater than chemical reactions. When uranium atoms split, they unleash energy that can flatten cities or power entire nations.
This power comes from the instability of uranium’s massive nucleus. Under the right conditions, a chain reaction occurs, where one nuclear fission triggers another, and another, in a cascading release of energy.
Uranium embodies humanity’s most complex relationship with elements. It represents both apocalypse and progress. Nuclear weapons reveal its destructive potential, while nuclear reactors demonstrate its ability to provide immense energy with minimal fuel.
Uranium is not just explosive. It is transformative. It changes landscapes, politics, and the future of civilization itself.
10. Cesium – The Most Explosive Metal You’ll Never Touch
Cesium is often considered the most explosively reactive metal known. It is so eager to react that it must be stored in sealed containers, isolated from air and moisture. Drop cesium into water, and the reaction is instantaneous and catastrophic, producing violent explosions and intense heat.
Cesium’s outer electron is held so loosely that the atom barely clings to it. The moment cesium encounters something that can accept that electron, the reaction is uncontrollable.
Because of this, cesium is rarely encountered outside controlled environments. Yet its extreme properties make it useful in atomic clocks, where its precise atomic behavior defines time itself.
Cesium’s personality is pure volatility. It cannot exist freely in the natural world. It must be restrained, isolated, and respected.
The Emotional Power of Explosive Elements
These ten elements reveal something profound about the universe. Matter is not passive. Atoms are not inert building blocks. They are dynamic entities with tendencies, desires, and limits shaped by the laws of physics.
Explosive personalities arise when atoms are pushed away from stability. When bonds are strained, electrons displaced, or nuclei destabilized, energy waits to be released. Sometimes that release builds stars. Sometimes it lights a candle. Sometimes it destroys cities.
Understanding these elements is not just an academic exercise. It is an act of humility. It reminds us that the forces we harness are ancient, powerful, and indifferent to human intentions. Science gives us the tools to understand them, but responsibility determines how they shape our world.
The periodic table is not just a chart. It is a map of personalities, tensions, and stories written in atomic fire.