Inside every cell of our body, a bustling world unfolds. Molecules flow, membranes shift, and organelles whisper to one another in a choreography so delicate that life itself depends on it. Among the many players in this dance, lipids—fat-like molecules—often go unnoticed. Yet lipids are far more than passive building blocks of cell membranes. They are versatile, dynamic, and exquisitely organized. Without them, there would be no cell division, no communication, no energy storage, no life as we know it.
But how do thousands of different lipid species, each with its own role, find their proper place within the crowded architecture of a cell? This question has haunted scientists for decades. Traditional imaging techniques could capture the broad strokes of lipid flow but not the precise routes or the split-second movements of individual species. Now, a groundbreaking study from the Max Planck Institute of Molecular Cell Biology and Genetics has shed light on this enigma, revealing the secret highways that lipids take as they dart between organelles.
Beyond the Vesicle: A New View of Lipid Transport
Until now, many scientists believed vesicles—tiny bubbles that shuttle cargo between compartments—were the main carriers of lipids inside cells. Vesicular trafficking is indeed important, but it is slow, non-specific, and limited by the machinery that pinches off and fuses membranes. The new research tells a different story: most lipids do not patiently ride inside vesicles at all. Instead, they travel through fast, selective, and direct routes—non-vesicular transport systems that can outpace vesicles by more than tenfold.
This discovery does not just refine our understanding of cell biology; it rewrites it. Imagine a bustling city. Vesicles are like delivery trucks navigating traffic-filled streets. Non-vesicular transport, by contrast, resembles express subways—direct, rapid, and targeted, ensuring that only the right molecules reach the right destination at precisely the right time.
The Experiment That Changed the Map
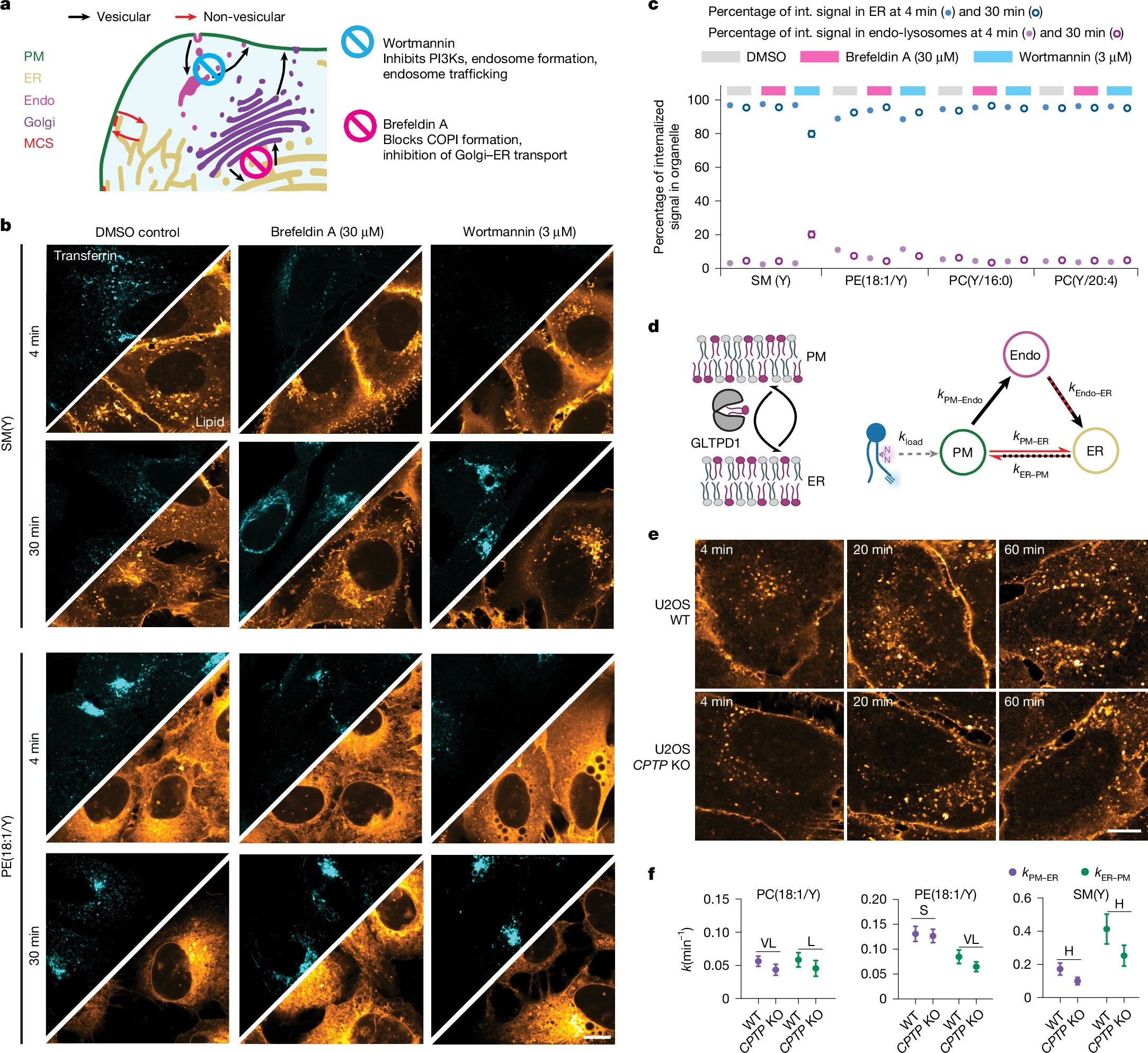
To uncover these hidden transport pathways, researchers developed a clever strategy. They designed “bifunctional lipid probes”—special lipid molecules equipped with chemical tags for both imaging and tracking. These probes were gently inserted into the plasma membrane of live human cells, then “locked in place” using ultraviolet light. Once anchored, the lipids’ journey could be followed in real time.
Using cutting-edge time-resolved fluorescence imaging and ultra-high-resolution mass spectrometry, the team mapped the lipids’ location and transformation. Mathematical models were layered on top of the data, comparing direct transfer routes with vesicular ones. To probe the molecular machinery involved, the researchers turned to genetic knockdowns and drug treatments, targeting proteins known to flip or transfer lipids across membranes.
The cells used were U2OS cells—a workhorse in biological research—supplemented by HCT116 cells for mechanistic tests. Together, these models revealed a detailed, quantitative atlas of retrograde lipid transport.
Lipids on the Move: Speed, Selectivity, and Surprises
The results were striking. Polyunsaturated species of phosphatidylcholine, phosphatidic acid, and phosphatidylethanolamine reached the endoplasmic reticulum (ER)—the cell’s central lipid factory—almost instantly. In contrast, saturated phosphatidylcholine and sphingomyelin tarried at the plasma membrane and in endosomes, their journeys slow and meandering.
Transport speed varied dramatically. Phosphatidylethanolamine was the speed champion, followed by polyunsaturated phosphatidylcholine and sphingomyelin, while saturated phosphatidylcholine lagged behind. Even subtle structural differences mattered: unsaturated phosphatidylcholines moved up to seven times faster than their saturated cousins, and lipids with chains positioned at the sn-2 position traveled up to twice as fast as those at sn-1.
Blocking vesicular trafficking with drugs did not halt the flow. Lipids still reached the ER, proving that non-vesicular routes dominate early transport. Yet the picture was nuanced. Sphingomyelin, for instance, piled up in endosomes when vesicular routes were blocked, showing that both systems work together, with each lipid species choosing its own path.
Knocking out specific proteins deepened the insight. Removing CPTP, a protein that ferries sphingomyelin, cut its ER delivery by nearly half. Silencing TMEM30A, a subunit of a lipid flippase, slowed phosphatidylethanolamine transport threefold and disrupted the balance between forward and backward flows.
Faster Than Chemistry Itself
One of the most surprising findings was that transport outpaces metabolism by an order of magnitude. Lipids moved between organelles ten to sixty times faster than they were chemically modified. Yet transport and metabolism were tightly linked: how a lipid moved influenced how it was later transformed. For example, sn-1-modified phosphatidylcholine generated more lipid droplets and cholesterol esters than sn-2 forms over longer timescales, highlighting how subtle structural details shape cellular outcomes.
This coupling suggests that transport is not merely about logistics—it is an active regulator of metabolism, dictating how energy is stored and how membranes evolve.
Why This Matters
The implications of this study ripple far beyond basic biology. Lipid metabolism is at the heart of many diseases, from neurodegeneration to metabolic syndrome to cancer. Understanding how lipids are sorted, transported, and transformed offers new opportunities to design therapies that target these pathways with unprecedented precision.
The research also provides an invaluable atlas—a kind of “molecular traffic map”—that will guide future studies in cell biology. It creates a foundation for mathematical modeling, drug discovery, and synthetic biology, offering researchers a tool to predict how cells reorganize their membranes in health and disease.
Most profoundly, the findings remind us of the elegance and complexity of life at its smallest scale. Beneath the surface of every cell lies a dynamic world where molecules race along invisible highways, orchestrated by forces we are only beginning to understand.
The Poetry of Lipids
Science often reveals beauty where we least expect it. Lipids may seem like humble fats, but in truth, they are more like artists, shaping the architecture of cells, painting the canvas of membranes, and choreographing the dance of organelles. Their journeys are not random; they are purposeful, swift, and exquisitely precise.
The Max Planck team’s work transforms how we view this hidden world. By tracing the secret highways of lipids, they have shown us that the vitality of life depends not just on molecules themselves, but on how they move, where they go, and how they interact along the way.
Science is often a story of uncovering the invisible. Here, the invisible is motion—the restless, purposeful movement of lipids that sustain every heartbeat, every thought, every breath. And now that we can see their journeys, we are one step closer to understanding the grand design of life itself.
More information: Juan M. Iglesias-Artola et al, Quantitative imaging of lipid transport in mammalian cells, Nature (2025). DOI: 10.1038/s41586-025-09432-x