Deep within the bustling environment of a living cell lies a structure that is often overlooked due to its elusive and understated presence—the nucleolus. Nestled inside the nucleus, like a secret chamber within a palace, the nucleolus is not encased by a membrane. Yet, despite its lack of a definitive boundary, this structure performs one of the most vital and complex tasks in cellular life: the production of ribosomes, the cell’s protein factories. Without the nucleolus, life would come to a screeching halt. Its influence stretches across every domain of life, touching everything from growth and development to aging and disease.
To appreciate the significance of the nucleolus is to uncover one of biology’s most elegant mechanisms—a symphony of molecular coordination, driven by the need to build the molecular machines that sustain life. In this article, we will journey through the biological intricacies and astonishing capabilities of the nucleolus, exploring its formation, structure, function, and its indispensable role in ribosome biogenesis. We will also peer into the latest scientific discoveries that shed light on its relevance to health and disease.
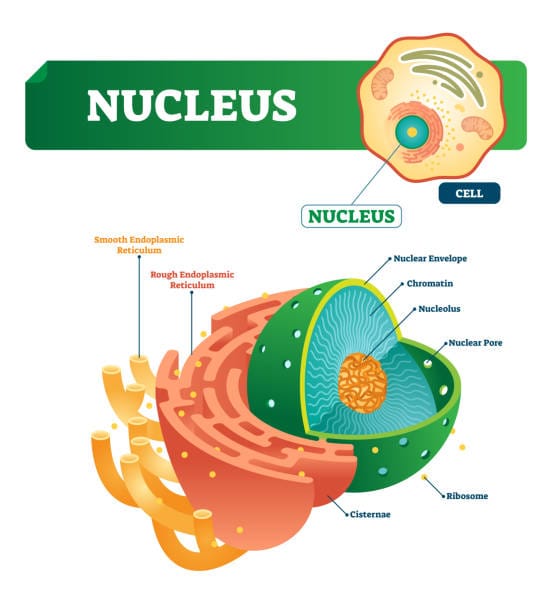
A Nucleus Within a Nucleus
The nucleolus is a dense, spherical region found within the nucleus of eukaryotic cells. Though prominent under a light microscope, it is not surrounded by a lipid membrane, unlike most organelles. Instead, it exists as a dynamic structure formed through a process known as liquid-liquid phase separation, much like oil droplets forming in water.
During interphase—the cell cycle phase when the cell is not dividing—the nucleolus is clearly visible. However, during mitosis (cell division), it disassembles and disperses, only to reassemble after division concludes. This cyclical disappearance and reappearance underscore its responsive and adaptable nature.
But what is it made of? The nucleolus is a composite of proteins, DNA, and RNA. It organizes around nucleolar organizer regions (NORs)—stretches of chromosomal DNA that contain genes encoding ribosomal RNA (rRNA). These regions are found on the short arms of several chromosomes in humans (specifically chromosomes 13, 14, 15, 21, and 22). Where these NORs exist, nucleoli form.
The nucleolus is not a homogeneous blob. It is an intricately structured and compartmentalized organelle, consisting of three main regions—fibrillar centers (FCs), dense fibrillar components (DFCs), and granular components (GCs)—each representing different stages of ribosome production.
Ribosomes: Builders of the Molecular World
To understand the gravity of the nucleolus’s function, we must first understand what ribosomes are and why they matter. Ribosomes are molecular machines responsible for synthesizing proteins from amino acids, using messenger RNA (mRNA) as a template. Proteins are the workhorses of the cell—responsible for nearly every task imaginable, from catalyzing biochemical reactions to forming structural elements.
Every second, ribosomes assemble proteins in a frenetic, rhythmic dance of molecular translation. Each ribosome is composed of two major subunits: a large subunit and a small subunit, which together contain ribosomal RNA (rRNA) and ribosomal proteins. These components must be precisely synthesized and assembled—a task orchestrated almost entirely within the nucleolus.
The importance of ribosomes cannot be overstated. They are essential for cell survival and proliferation. Cells that are highly active—like liver cells, cancer cells, or stem cells—often have larger and more numerous nucleoli due to their increased need for protein synthesis. Conversely, in aging or diseased cells, nucleolar function can become impaired, with serious consequences.
The Blueprint: Transcription of rRNA
The first major role of the nucleolus is the transcription of ribosomal RNA genes. These genes are among the most actively transcribed in the genome. In humans, there are approximately 400 copies of rRNA genes, and they are transcribed by RNA polymerase I, an enzyme dedicated almost exclusively to rRNA synthesis.
This transcription occurs in the fibrillar centers and dense fibrillar components of the nucleolus. The DNA templates—called rDNA—are transcribed into a single, large precursor molecule: the 47S pre-rRNA. This long strand contains the blueprints for the three main types of rRNA used in ribosomes: 18S, 5.8S, and 28S rRNA.
Notably, the 5S rRNA, a component of the large ribosomal subunit, is not transcribed in the nucleolus but elsewhere in the nucleus by RNA polymerase III, and is later imported into the nucleolus for assembly.
The transcription process is tightly regulated. It involves a variety of transcription factors, co-factors, and chromatin-remodeling complexes that ensure only the right genes are activated, and at the right time. Growth factors, nutrient availability, and stress signals can all influence the rate of rRNA transcription, making the nucleolus a responsive center for environmental adaptation.
Processing the rRNA: Molecular Refinement
Once transcribed, the 47S pre-rRNA must undergo extensive processing to become functional. This process is highly coordinated and includes cleavage, chemical modification, and folding of the RNA molecule.
Key chemical modifications include methylation of ribose sugars and pseudouridylation (conversion of uridine to pseudouridine), which stabilize the rRNA structure and enhance its functional capacity. These modifications are not random—they are guided by small nucleolar RNAs (snoRNAs), which act like GPS coordinates for molecular editors.
As the pre-rRNA is processed, it is cleaved into its constituent parts: 18S rRNA (for the small subunit) and 5.8S and 28S rRNA (for the large subunit). Each of these steps occurs in different regions of the nucleolus—mainly transitioning into the granular component, where early assembly of ribosomal subunits begins.
Assembly Line: Birth of Ribosomal Subunits
Ribosome assembly is one of the most energy-intensive and complex processes in the cell. It requires the coordinated action of hundreds of proteins and dozens of snoRNAs. The processed rRNA molecules are combined with ribosomal proteins, which are synthesized in the cytoplasm and imported into the nucleus.
The small ribosomal subunit (40S) is formed around the 18S rRNA, while the large subunit (60S) contains the 5.8S, 28S, and imported 5S rRNAs. These subunits are not yet functional. They must undergo quality control checks and further maturation before being exported to the cytoplasm.
Special transport factors recognize the mature ribosomal subunits and escort them through the nuclear pore complexes into the cytoplasm. There, the two subunits remain separate until they are needed for translation. When an mRNA arrives, the ribosome assembles around it and begins the process of protein synthesis.
Beyond Ribosomes: Nucleolus as a Sensor and Regulator
Although ribosome production is the nucleolus’s primary function, it is far from being its only one. Recent research has revealed that the nucleolus is a central hub for cellular stress response, cell cycle regulation, aging, and disease pathology.
The nucleolus can sense various forms of cellular stress—such as DNA damage, oxidative stress, or nutrient deprivation—and alter its activity accordingly. During such events, rRNA transcription slows down, ribosome production decreases, and cell cycle arrest may be triggered. This regulation involves p53, the well-known tumor suppressor protein. Under stress, nucleolar proteins like nucleophosmin (NPM1) and ARF interact with p53 to stabilize it and prevent damaged cells from dividing.
The nucleolus also plays a role in telomerase assembly, RNA processing, and even viral replication, with some viruses hijacking nucleolar components to promote their life cycles. Its involvement in so many pathways has earned the nucleolus the reputation of being more than just a ribosome factory—it is a multifunctional command center.
When the Nucleolus Fails: Disease and Dysfunction
Given its central role, it’s no surprise that nucleolar dysfunction is implicated in a variety of diseases. Cancer is among the most prominent. Cancer cells often have enlarged and hyperactive nucleoli, reflecting their high demand for protein synthesis. Increased nucleolar size has even been used as a diagnostic marker in certain tumors.
Moreover, some cancers result from mutations in genes involved in ribosome production or rRNA processing. These conditions are part of a growing group of disorders known as ribosomopathies, which also include congenital diseases like Diamond-Blackfan anemia and Treacher Collins syndrome. In these conditions, defective ribosome production leads to specific developmental abnormalities, often in bone marrow, craniofacial structures, or the immune system.
Even aging is linked to nucleolar activity. Studies have shown that reduced nucleolar size and activity are associated with increased lifespan in various organisms. This paradox—less ribosome production leading to longer life—suggests a delicate balance between growth and maintenance. The nucleolus, it seems, is at the heart of this balance.
Peering Through the Microscope: Tools of Discovery
Modern techniques have allowed scientists to study the nucleolus with increasing resolution and precision. Electron microscopy reveals its layered ultrastructure. Fluorescence microscopy can track nucleolar proteins and RNA in real time. Chromatin immunoprecipitation (ChIP) and next-generation sequencing (NGS) have uncovered regulatory mechanisms governing rRNA gene expression.
Mass spectrometry is used to analyze the proteome of the nucleolus, while live-cell imaging tracks ribosome assembly dynamics. Scientists now use techniques like proximity labeling and single-molecule RNA imaging to uncover interactions at the molecular level. As we peer deeper into this organelle, its complexity continues to amaze.
A Glimpse into the Future
The study of the nucleolus is far from complete. New questions arise every year: How exactly does the nucleolus influence aging? Can we target it in cancer therapy without harming normal cells? Could nucleolar components serve as biomarkers for early disease detection?
One of the most promising avenues is the development of nucleolus-targeting drugs. Inhibitors of RNA polymerase I are being tested as anti-cancer agents. If we can selectively dial down ribosome production in tumor cells, we might slow their growth or induce cell death. But the challenge remains to preserve healthy tissue and minimize side effects.
Researchers are also exploring the nucleolus’s role in neurodegenerative diseases, including Alzheimer’s and Parkinson’s. Misfolded proteins and impaired RNA metabolism often involve the nucleolus, though the precise mechanisms remain elusive.
The Beating Heart of the Nucleus
In the grand orchestra of cellular life, the nucleolus is a conductor whose influence resonates in every note played by the ribosomes. It bridges the gap between genome and proteome, between genetic potential and biochemical reality. It is a testament to nature’s ability to create order from chaos, complexity from simplicity.
Though it lacks a membrane, the nucleolus possesses an identity as vivid and essential as any other organelle. It stands as a symbol of the hidden complexity that governs life at the microscopic level—a complexity that scientists are still unraveling, strand by strand, ribosome by ribosome.
In learning about the nucleolus, we gain not just insight into biology, but a deeper appreciation for the molecular miracles that sustain every heartbeat, every breath, and every moment of our conscious experience.