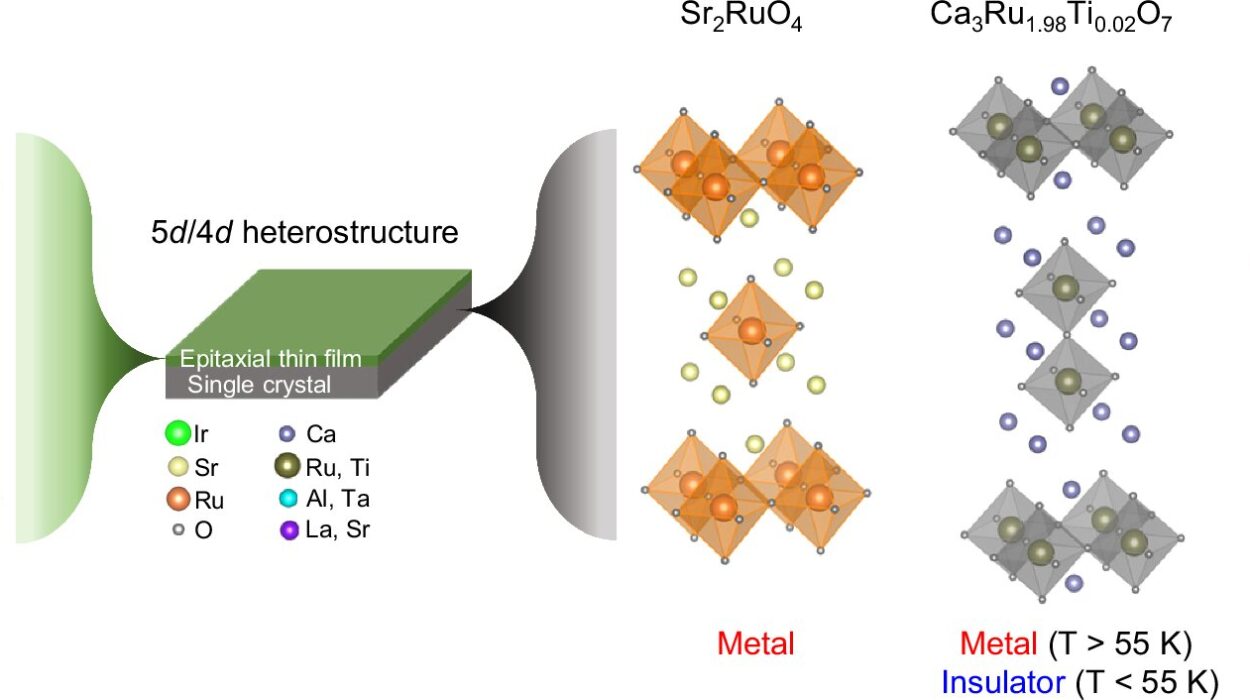
Crystals have fascinated humanity for millennia. From sparkling gemstones treasured in jewelry to the ice crystals that form delicate snowflakes, their beauty is undeniable. But beneath their dazzling exterior lies a world of precise order—a microscopic symphony of atoms arranged in repeating patterns that define the essence of a crystal. The physics of crystals unveils the extraordinary way matter organizes itself at the atomic scale, revealing principles that connect nature’s elegance with fundamental laws of physics.
In this article, we embark on a journey deep into the atomic landscape of crystals. We will explore what defines a crystal, how atoms align in intricate structures, the forces that maintain these patterns, and the remarkable properties that arise from such order. Along the way, we’ll meet the pioneers who unlocked these secrets, discover the cutting-edge applications born from crystal physics, and glimpse the frontier of research where quantum phenomena and crystal structures intertwine.
What Is a Crystal? The Foundation of Order
At its core, a crystal is a solid material whose atoms are arranged in a highly ordered, repeating pattern extending in all three spatial dimensions. This periodicity distinguishes crystals from amorphous solids like glass, where atoms are arranged randomly without long-range order.
The term “crystal” comes from the Greek word krustallos, meaning “ice” or “frozen,” reflecting early observations of the symmetrical shapes of ice and salt crystals. When you look closely at a crystal, its external geometric shape—the facets and angles you see—reflects the internal symmetry of its atomic arrangement. This harmony between internal and external order is one of the marvels of crystallography.
Unlike everyday solids that may appear solid but are disordered internally, crystals exhibit long-range translational symmetry. Imagine an atomic pattern that, if shifted by certain vectors in space, would exactly overlap itself—this is the essence of a crystal lattice. The smallest repeating unit that contains all the atomic information is called the unit cell, and by stacking this unit cell repeatedly, the entire crystal is built.
The study of these ordered atomic patterns is called crystallography, a field that combines physics, chemistry, and mathematics to decode the structural blueprints of matter. It’s the physics of this repeating order that gives crystals their unique mechanical, optical, electrical, and thermal properties.
The Building Blocks: Atoms, Ions, and Molecules in Crystals
To understand crystals, we must first consider the atoms themselves. Atoms are the fundamental units of matter, each composed of a nucleus surrounded by electrons. When atoms come together to form solids, their electrons and nuclei interact via forces governed by quantum mechanics and electromagnetism.
Crystals can form from pure elements—like diamond, which is a crystal of carbon atoms—or from compounds, such as sodium chloride (table salt), where sodium and chloride ions alternate in a cubic pattern. Molecules, too, can crystallize, forming organic crystals such as sugar or complex biological molecules like proteins.
The arrangement of these building blocks depends on the balance of forces. Attractive forces, such as ionic or covalent bonds, pull atoms together, while repulsive forces, including electron cloud overlap and thermal vibrations, push them apart. The crystal structure settles into the configuration that minimizes the total energy—a delicate equilibrium known as the ground state.
This equilibrium leads to the most stable arrangement of atoms. For example, in sodium chloride, the sodium ions (Na⁺) and chloride ions (Cl⁻) arrange themselves in a cubic lattice, maximizing attraction between oppositely charged ions and minimizing repulsion between like charges.
Crystal Lattices and Unit Cells: The Blueprint of Order
Imagine standing inside a crystal and looking around. You would see atoms arranged in a periodic three-dimensional grid extending infinitely. This grid is the crystal lattice, an abstract mathematical array of points representing atomic positions.
Each point in the lattice corresponds to an identical environment—a precise atomic neighborhood repeated throughout the solid. The smallest building block that, when repeated through space by translation, recreates the entire lattice is the unit cell. The unit cell is defined by three vectors and the angles between them, which together determine the crystal’s symmetry and geometry.
The seven crystal systems—cubic, tetragonal, orthorhombic, hexagonal, trigonal (rhombohedral), monoclinic, and triclinic—classify crystals by the shapes of their unit cells and the symmetry they exhibit. For example, cubic crystals have equal edges and right angles, exemplified by salt or diamond, while monoclinic crystals have edges and angles that differ, as seen in some minerals.
More intricate still is the concept of the Bravais lattices, named after Auguste Bravais, who showed in 1848 that there are only 14 distinct three-dimensional lattice types that can fill space without gaps. These lattice types underpin all crystal structures.
Within each lattice, atoms occupy specific sites, arranged in patterns characteristic of the material. The basis refers to the group of atoms associated with each lattice point, and the combination of the lattice plus basis fully describes the crystal structure.
Symmetry and Crystallography: The Language of Patterns
Symmetry lies at the heart of crystal physics. It is the tool scientists use to classify and understand crystal structures. Symmetry describes operations—like rotations, reflections, and translations—that map a structure onto itself without changing its appearance.
Crystals exhibit various symmetry elements: rotational axes, mirror planes, inversion centers, and glide planes. These symmetries form groups called space groups, which describe all possible symmetry arrangements in three-dimensional crystals. There are 230 unique space groups recognized in crystallography.
Understanding symmetry helps predict and explain physical properties. For instance, certain symmetries forbid or allow phenomena like piezoelectricity—the generation of electric charge under mechanical stress. Only crystals lacking a center of symmetry can exhibit piezoelectric effects.
Forces Holding Crystals Together: The Interatomic Glue
The structure of a crystal is not just a geometric curiosity; it is the result of physical forces acting between atoms. Several types of bonding mechanisms govern crystal formation and stability:
Ionic Bonds: Formed between positively and negatively charged ions, ionic bonds are strong electrostatic attractions. Crystals like sodium chloride rely on ionic bonds to hold their alternating positive and negative ions in a stable lattice.
Covalent Bonds: Atoms share electron pairs to achieve stability in covalent bonds. Diamond is a prime example, where carbon atoms form a rigid three-dimensional network through strong covalent bonds, resulting in extreme hardness.
Metallic Bonds: In metals, atoms release some electrons to form a “sea” of delocalized electrons that flow freely, binding the positively charged metal ions. This gives metals their conductivity and malleability.
Van der Waals Forces: Weaker than covalent or ionic bonds, these forces arise from induced dipoles in atoms or molecules. They play a significant role in molecular crystals like solidified noble gases or organic compounds.
Hydrogen Bonds: A special type of dipole interaction involving hydrogen atoms bonded to electronegative atoms, hydrogen bonds help hold biological crystals such as ice or proteins together.
The interplay of these bonding types determines the crystal’s mechanical strength, melting point, electrical behavior, and more.
Crystal Growth: From Atoms to Macroscopic Solids
Crystals often grow from solutions, melts, or vapors when conditions such as temperature and concentration favor solidification. Crystal growth is a complex process governed by kinetics and thermodynamics.
At the atomic level, growth begins with nucleation, where a small cluster of atoms forms a stable “seed” that can grow into a full crystal. Once nucleation occurs, atoms from the surrounding environment attach to the crystal surface in an orderly manner, extending the lattice.
The growth rate and shape depend on factors like temperature, supersaturation, and impurities. Slow growth typically produces well-formed, large crystals with smooth faces, while rapid growth can lead to irregular shapes or polycrystalline aggregates.
Understanding crystal growth is crucial in industries ranging from semiconductor manufacturing, where silicon crystals form the backbone of electronics, to pharmaceuticals, where the size and shape of drug crystals affect their dissolution and efficacy.
X-Ray Diffraction: Peering Inside Crystals
One of the greatest breakthroughs in understanding crystal structure came with the discovery of X-ray diffraction. In 1912, physicists Max von Laue and the Braggs—William Henry and William Lawrence—realized that when X-rays pass through a crystal, the regular atomic arrangement causes the X-rays to scatter in specific patterns.
By analyzing these diffraction patterns, scientists can reconstruct the three-dimensional positions of atoms within the crystal—a technique called X-ray crystallography. This method has unveiled the structures of countless materials, from minerals to complex biological molecules like DNA.
X-ray crystallography revolutionized physics, chemistry, and biology by providing a window into the atomic world and enabling precise understanding of material properties.
Physical Properties Emerging from Crystal Structure
The ordered arrangement of atoms in crystals gives rise to unique physical properties that differ drastically from amorphous solids or liquids. The symmetry, bonding, and lattice parameters dictate how crystals behave under various conditions.
For example, electrical conductivity in metals arises because of the periodic lattice allowing free movement of electrons. Semiconductors, with their precise crystal lattices and controlled impurities, underpin modern electronics.
Optical properties such as birefringence occur in anisotropic crystals, where light velocity varies with direction due to differing atomic arrangements. Piezoelectricity, found in quartz crystals, allows mechanical stress to generate electric charge, powering sensors and actuators.
Thermal conductivity also depends on lattice vibrations or phonons—the collective oscillations of atoms. Perfect crystal lattices conduct heat efficiently, whereas defects and disorder scatter phonons and reduce conductivity.
Defects in Crystals: Imperfections with Purpose
No crystal is perfect. Real crystals contain defects—imperfections in the otherwise regular atomic arrangement. These defects profoundly influence material properties.
Point defects include vacancies (missing atoms) and interstitials (extra atoms squeezed in). Line defects, called dislocations, are disruptions along a row of atoms that allow crystals to deform plastically without breaking.
Surface defects and grain boundaries—interfaces between differently oriented crystal domains—also affect mechanical strength and electrical behavior.
While defects often reduce material performance, they are also exploited intentionally. Semiconductor doping introduces specific defects to control electrical conductivity, essential for transistors and diodes.
Crystals at the Quantum Frontier
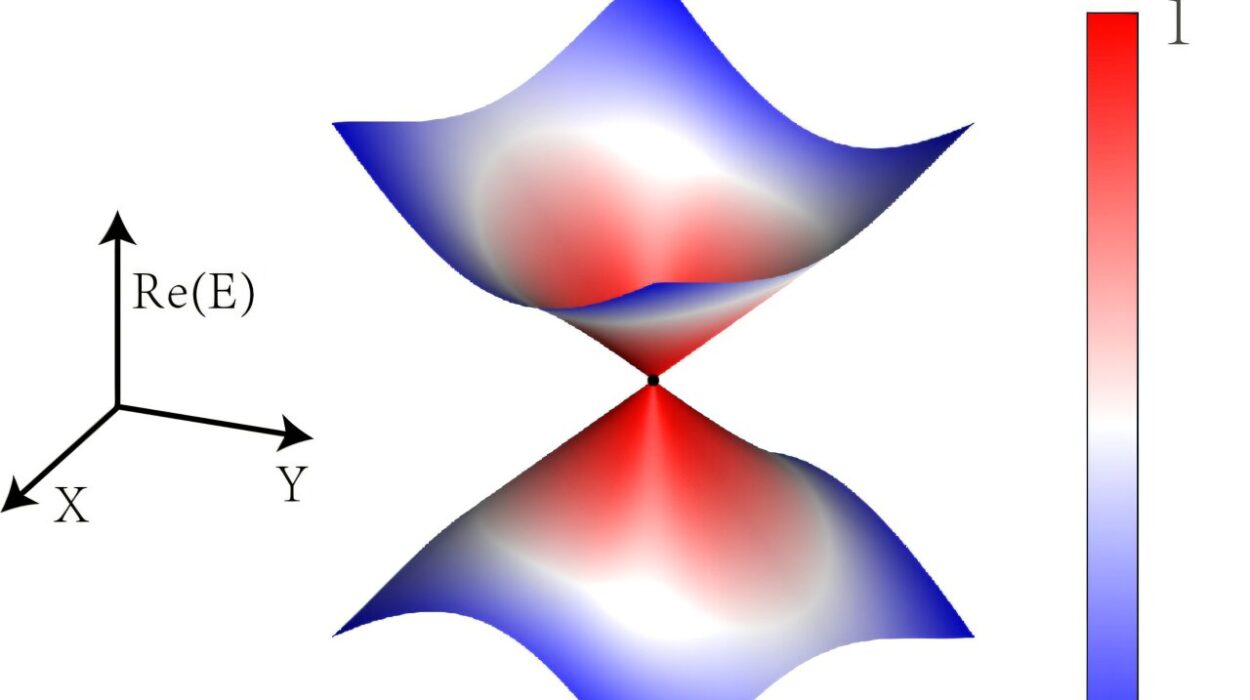
At the smallest scales, the physics of crystals intersects with quantum mechanics. The periodic lattice profoundly influences the behavior of electrons, leading to phenomena like energy bands and band gaps. This understanding forms the basis of solid-state physics.
The concept of Bloch waves describes how electrons propagate through a crystal lattice, explaining electrical conductivity and insulating behavior. Band theory reveals why some crystals conduct electricity while others act as insulators or semiconductors.
More recently, research into topological insulators and quantum crystals explores exotic states of matter where quantum effects at the atomic scale lead to robust surface states immune to defects, promising revolutionary advances in quantum computing and electronics.
Crystals Beyond Earth: Natural and Synthetic Marvels
Crystals are not confined to Earth. In the vastness of space, crystals form in meteorites, planetary interiors, and the icy moons of the outer solar system. The conditions of extreme pressure and temperature yield exotic crystal forms, including superionic ice and metallic hydrogen.
On Earth, synthetic crystals are engineered with incredible precision. From ultrapure silicon for computer chips to synthetic diamonds used in cutting tools and quantum devices, human ingenuity leverages crystal physics to push technology forward.
Biological crystals, too, reveal nature’s sophistication. Protein crystallography enables drug design, while biomineralization processes produce shells and bones with remarkable mechanical properties.
The Future of Crystal Physics
As we advance into the 21st century, the physics of crystals continues to be a vibrant frontier. New materials such as graphene—a single layer of carbon atoms arranged in a honeycomb lattice—challenge traditional paradigms with exceptional strength, conductivity, and flexibility.
Two-dimensional crystals, quantum dots, and metamaterials expand the possibilities for controlling light, heat, and electrons with unprecedented precision. Techniques like ultrafast spectroscopy and electron microscopy allow real-time observation of atomic motion during phase transitions.
The quest for room-temperature superconductors—a holy grail of physics—relies on understanding how crystal structures facilitate or hinder electron pairing and resistance-free current flow.
Crystals remain a testament to the fundamental principle that order on the smallest scales shapes the universe we experience. From the beauty of a snowflake to the silicon chips powering our digital lives, the physics of crystals connects us to the atomic dance that underpins reality itself.