The Earth is enveloped by an invisible shield that protects life from the harsh realities of outer space. This shield is not made of metal or energy fields but of gases—delicate, dynamic layers of the atmosphere that regulate temperature, absorb harmful radiation, and sustain the planet’s intricate balance. Among these layers, the ozone layer plays one of the most critical roles. It filters ultraviolet radiation from the Sun, ensuring that life can thrive without being scorched by deadly rays. Yet, beyond the ozone, the Sun’s constant stream of energy and charged particles—collectively known as solar radiation and space weather—shapes the very environment in which Earth exists.
Understanding how these forces interact reveals not just the fragility of our planet’s systems but also their remarkable resilience. The ozone layer, solar radiation, and space weather are interlinked elements of a grand cosmic interplay, binding our planet to its star in both harmony and hazard.
The Structure of Earth’s Atmosphere
Before exploring the ozone layer in depth, it is essential to understand where it resides. The Earth’s atmosphere is divided into several layers, each defined by changes in temperature and composition. Closest to the surface lies the troposphere, the region where weather occurs and where nearly all atmospheric water vapor is found. Above it is the stratosphere, extending from roughly 10 to 50 kilometers above the surface.
The ozone layer lies within the stratosphere, primarily between 15 and 35 kilometers above Earth. Above the stratosphere lies the mesosphere, then the thermosphere, and finally the exosphere, which gradually fades into space. Each layer plays a distinct role in maintaining planetary stability, but the stratosphere—home to the ozone layer—is where sunlight and chemistry combine to create one of the planet’s most vital defense mechanisms.
What the Ozone Layer Is Made Of
Ozone is a molecule composed of three oxygen atoms (O₃). It forms when ordinary oxygen molecules (O₂) are exposed to ultraviolet (UV) light from the Sun. In this process, known as photodissociation, UV radiation splits an oxygen molecule into two oxygen atoms, which then combine with other oxygen molecules to form ozone.
This simple yet elegant chemical reaction creates a dynamic equilibrium: ozone continuously forms and breaks down under the influence of sunlight. This balance maintains a relatively steady concentration of ozone in the stratosphere, allowing it to absorb ultraviolet radiation effectively.
Most of the ozone in the atmosphere is found between 15 and 30 kilometers altitude. Even so, if all the ozone in the atmosphere were compressed to standard surface pressure, it would form a layer only about three millimeters thick—proof that even thin barriers can have profound importance.
How the Ozone Layer Protects Life
The Sun emits radiation across a vast spectrum, from radio waves to gamma rays. Of this radiation, ultraviolet light is particularly dangerous to living organisms. UV radiation is divided into three main types based on wavelength: UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm).
UV-C radiation is the most energetic and destructive, capable of breaking molecular bonds and damaging DNA directly. Fortunately, the ozone layer absorbs nearly all UV-C radiation and most UV-B. Without this filtering effect, life as we know it could not exist on land.
Even small changes in ozone concentration can significantly affect the amount of UV radiation reaching the surface. Increased UV-B exposure leads to higher risks of skin cancer, cataracts, and immune system suppression in humans, while also damaging phytoplankton, crops, and terrestrial ecosystems. The ozone layer thus acts as Earth’s sunscreen, modulating solar energy to create conditions suitable for biological evolution.
The Discovery and Study of Ozone
The story of ozone is one of scientific curiosity and global collaboration. In the mid-19th century, Christian Friedrich Schönbein first identified ozone as a distinct chemical compound, naming it after the Greek word “ozein,” meaning “to smell,” due to its sharp odor after lightning storms.
By the early 20th century, French physicists Charles Fabry and Henri Buisson discovered that ozone concentrated in the upper atmosphere. Later, British scientist G. M. B. Dobson developed the first instrument capable of measuring atmospheric ozone from the ground. His spectrophotometer allowed systematic monitoring of ozone levels worldwide, leading to the unit of measurement known as the Dobson Unit (DU). A typical ozone layer thickness ranges between 250 and 500 DU, varying with latitude and season.
These early discoveries laid the foundation for understanding how human activity could alter this delicate layer—a realization that would emerge dramatically in the late 20th century.
The Ozone Hole and Human Impact
In the 1970s, scientists Mario Molina and F. Sherwood Rowland uncovered a troubling possibility. They theorized that chlorofluorocarbons (CFCs)—chemicals used in refrigeration, aerosols, and industrial processes—could rise into the stratosphere and destroy ozone molecules. Under intense UV radiation, CFCs release chlorine atoms, which act as catalysts in reactions that break apart ozone. A single chlorine atom can destroy thousands of ozone molecules before being neutralized.
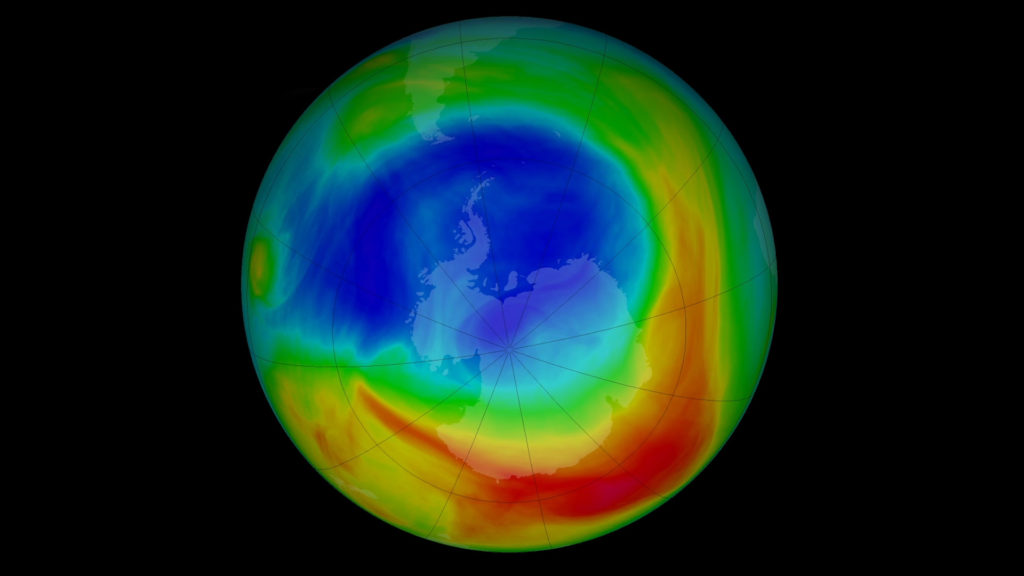
In 1985, British researchers from the British Antarctic Survey reported a dramatic seasonal depletion of ozone over Antarctica. This “ozone hole” formed each spring when sunlight returned after the polar night, triggering chemical reactions on the surface of icy stratospheric clouds that accelerated ozone destruction.
The global response to this crisis was unprecedented. In 1987, the Montreal Protocol was adopted, committing nations to phase out CFCs and related substances. Over the following decades, the treaty became one of the most successful environmental agreements in history, demonstrating that international cooperation could reverse large-scale environmental damage.
Today, the ozone layer is gradually recovering, though full restoration is expected to take until the mid-21st century. The story of the ozone hole remains a vital example of how human activity can alter atmospheric chemistry—and how science and policy can work together to correct it.
Solar Radiation: The Lifeblood and Threat of Earth
The Sun is both the giver and taker of life. Every second, it emits around 3.8 × 10²⁶ watts of energy, most of which radiates into space. A fraction of that energy reaches Earth, driving photosynthesis, weather patterns, and the climate system. Yet, this same radiation contains the potential for destruction if not moderated by Earth’s natural shields.
Solar radiation encompasses a wide range of electromagnetic wavelengths. Visible light warms the surface and sustains life, while infrared radiation regulates planetary temperature. Ultraviolet radiation, on the other hand, poses biological risks but also contributes to chemical processes such as ozone formation.
The balance of incoming and outgoing solar energy defines Earth’s climate. Roughly one-third of solar radiation is reflected back into space by clouds, aerosols, and surface ice, while the remainder is absorbed by land, oceans, and atmosphere. This absorbed energy is re-emitted as infrared radiation, maintaining the planet’s energy equilibrium. When greenhouse gases trap excess infrared radiation, this balance shifts, leading to global warming.
The Sun’s Variable Nature
The Sun is not a constant source of radiation. It undergoes an 11-year solar cycle characterized by fluctuations in magnetic activity, visible through sunspots and solar flares. During periods of high activity—solar maxima—the Sun emits greater amounts of ultraviolet radiation and releases powerful bursts of charged particles known as solar wind.
These variations can influence the ozone layer and Earth’s upper atmosphere. Increased UV output during solar maxima enhances ozone production, while charged particles from solar storms can deplete ozone locally by interacting with atmospheric molecules. Though these effects are generally temporary, they highlight the Sun’s direct influence on atmospheric chemistry and climate dynamics.
The Sun also emits bursts of high-energy particles in solar flares and coronal mass ejections (CMEs). When directed toward Earth, these events can disrupt satellites, power grids, and radio communications—a domain known as space weather.
What Is Space Weather?
Space weather refers to the dynamic conditions in the near-Earth space environment influenced by solar activity. It encompasses phenomena such as solar flares, geomagnetic storms, and energetic particle events. These disturbances originate from the Sun’s magnetic field, which constantly interacts with the interplanetary medium and Earth’s magnetosphere.
The solar wind—a continuous stream of charged particles emitted by the Sun—travels through space at speeds of 300 to 800 kilometers per second. When it encounters Earth’s magnetic field, it distorts and compresses it on the sunward side, forming a protective bubble known as the magnetosphere. The magnetosphere deflects most solar particles, but during intense solar events, some penetrate deeper, generating geomagnetic storms.
These storms can induce electric currents in the atmosphere and ground, affecting satellites, communication systems, navigation signals, and power grids. The vivid auroras seen near polar regions—known as the aurora borealis and aurora australis—are visual manifestations of these interactions, caused when energetic particles excite atmospheric gases.
The Magnetosphere: Earth’s Invisible Shield
The magnetosphere is Earth’s first line of defense against space weather. Generated by the movement of molten iron in the planet’s outer core, Earth’s magnetic field extends tens of thousands of kilometers into space. It channels and redirects charged particles, preventing most from reaching the atmosphere directly.
Without the magnetosphere, the relentless solar wind would strip away Earth’s atmosphere, as it has done on Mars. The magnetosphere not only protects atmospheric gases but also reduces radiation exposure for both the biosphere and technological systems.
However, the magnetosphere is not impervious. When solar storms are strong enough, magnetic reconnection occurs—where the magnetic field lines of the Sun and Earth realign explosively, allowing charged particles to pour into the upper atmosphere. This process triggers geomagnetic storms that can disrupt human technology and atmospheric chemistry alike.
Space Weather and Atmospheric Chemistry
The influence of space weather extends beyond electronics and communications. High-energy particles penetrating the upper atmosphere can ionize nitrogen and oxygen molecules, forming reactive compounds such as nitric oxides. These compounds can descend into the stratosphere, where they participate in chemical reactions that destroy ozone.
This effect is particularly pronounced near the poles, where the magnetic field funnels particles downward. During strong solar proton events, localized ozone depletion can reach up to 20–40 percent, although these reductions are usually temporary and recover within weeks. Nonetheless, frequent or prolonged events could have cumulative impacts on climate and radiation exposure.
Space weather also interacts with the atmosphere’s thermal structure. Energetic particle precipitation alters temperature and density in the mesosphere and thermosphere, influencing satellite orbits and radio wave propagation. Understanding these dynamics is essential for both atmospheric science and space operations.
Climate Connections Between the Sun and Earth
Solar variability influences climate over both short and long timescales. Over the 11-year solar cycle, changes in UV radiation can alter stratospheric ozone levels, affecting the temperature gradient between atmospheric layers and shifting wind patterns. These subtle shifts cascade downward, modifying jet streams and weather patterns in the troposphere.
Historically, prolonged periods of low solar activity—such as the Maunder Minimum between 1645 and 1715—coincided with cooler global temperatures, known as the Little Ice Age. Although such correlations do not imply direct causation, they demonstrate the Sun’s potential to influence Earth’s climate system.
Modern climate change, however, is driven primarily by anthropogenic greenhouse gas emissions rather than solar variability. While the Sun’s output fluctuates by less than one-tenth of one percent across solar cycles, human-induced forcing from carbon dioxide and methane has produced far greater radiative imbalance. Nonetheless, understanding solar contributions remains vital for accurate climate modeling.
The Coupling of Atmospheric Layers
The ozone layer, solar radiation, and space weather form a tightly coupled system linking all atmospheric layers. Energy from the Sun drives photochemical reactions in the stratosphere, while geomagnetic activity injects energy into the thermosphere. Between these regions, waves and currents transport heat and momentum, connecting the lower atmosphere to space.
This vertical coupling means that disturbances in one layer can influence others. For instance, strong geomagnetic storms heat the upper atmosphere, increasing density and drag on satellites. Simultaneously, ozone variations in the stratosphere can affect surface weather by altering circulation patterns. The atmosphere is not a collection of isolated strata but a continuous, responsive system shaped by both terrestrial and extraterrestrial forces.
The Role of Satellites and Observations
Modern science monitors these complex interactions through a network of satellites, ground-based observatories, and computational models. Instruments like NASA’s Aura, ESA’s Sentinel-5P, and the Solar Dynamics Observatory provide continuous data on ozone concentration, solar emissions, and geomagnetic activity.
Ground-based systems, including neutron monitors and magnetometers, complement satellite observations, detecting cosmic rays and geomagnetic fluctuations. Together, these systems form the foundation of space weather forecasting, allowing scientists to predict solar storms and mitigate their effects on infrastructure.
For example, when a coronal mass ejection is detected on the Sun, forecasters can estimate its arrival time at Earth—typically 1 to 3 days later—and issue alerts to satellite operators and power grid managers. Such foresight is critical in preventing cascading failures during geomagnetic disturbances.
Space Weather and Technological Vulnerability
The technological world depends on satellites, communication networks, and electrical grids—all of which are vulnerable to space weather. During severe geomagnetic storms, induced currents can overload transformers, leading to widespread blackouts. The most famous example occurred in 1989, when a powerful solar storm caused the collapse of Quebec’s power grid in seconds.
Satellites face multiple hazards: increased atmospheric drag during geomagnetic storms can cause orbital decay, while energetic particles can damage electronic components and solar panels. High-frequency radio communication and GPS navigation also degrade during solar events due to ionospheric disturbances.
As human presence in space expands, understanding and mitigating space weather becomes even more vital. Astronauts aboard the International Space Station or future lunar missions face radiation exposure that can exceed safe limits during solar particle events. Accurate forecasting and protective measures are thus essential for crew safety.
Long-Term Effects and Planetary Comparisons
The relationship between solar radiation and atmospheric protection extends beyond Earth. Mars and Venus provide instructive contrasts. Mars, with its weak magnetic field and thin atmosphere, is exposed directly to the solar wind, leading to gradual atmospheric erosion. Venus, though it lacks a global magnetic field, has a dense atmosphere that absorbs solar energy differently, resulting in extreme surface temperatures and a runaway greenhouse effect.
These planetary examples underscore the delicate balance that sustains Earth’s habitability. The combination of a robust magnetic field, a stable ozone layer, and an atmosphere rich in oxygen and nitrogen allows Earth to deflect, absorb, and regulate solar energy effectively.
Studying how solar radiation interacts with other planets helps scientists predict the long-term evolution of atmospheres and assess habitability elsewhere in the universe.
Emerging Research and Future Challenges
Research into the ozone layer, solar radiation, and space weather continues to advance. Scientists now study how climate change and ozone recovery interact, as greenhouse gases cool the stratosphere, influencing ozone chemistry and circulation. While the Montreal Protocol curbed CFC emissions, new compounds like hydrofluorocarbons (HFCs) raise concerns due to their global warming potential.
At the same time, solar observation missions such as the Parker Solar Probe and Solar Orbiter are uncovering the mysteries of the Sun’s magnetic field and solar wind dynamics. Understanding how these processes operate will refine space weather prediction models and improve resilience against future disruptions.
The integration of machine learning and big data analytics allows real-time analysis of solar and atmospheric data, enhancing forecasting accuracy. As societies depend increasingly on digital infrastructure, early warning systems for solar storms will become as essential as weather forecasts.
Human Adaptation and Planetary Stewardship
The story of the ozone layer and space weather is ultimately one of balance and adaptation. Humanity’s discovery of the ozone hole and collective response proved that global cooperation can repair planetary damage. Yet, the same spirit of vigilance must extend to new challenges—climate change, solar variability, and space environment hazards.
Protecting Earth’s atmospheric shields is not a one-time task but an ongoing responsibility. Every technological advance that pushes the boundaries of exploration—whether launching satellites, colonizing Mars, or harnessing solar energy—must respect the interconnected systems that sustain life on our home planet.
As we reach further into space, the lessons of the ozone layer remind us that even invisible layers of gas can determine the fate of worlds. The same Sun that gives life also tests resilience, illuminating both the beauty and fragility of Earth’s cosmic existence.
Conclusion
The ozone layer, solar radiation, and space weather form a triad of interdependence that defines Earth’s relationship with the Sun. The ozone layer filters radiation essential for life yet dangerous in excess. Solar radiation powers climate and ecosystems but fluctuates in ways that can threaten stability. Space weather, born of solar storms and magnetic turbulence, connects our planet’s atmosphere to the vast dynamics of the heliosphere.
Together, these forces shape every breath of air, every ray of sunlight, and every spark of technology on Earth. Their study is not merely scientific but existential, reminding us that the conditions for life depend on fragile balances maintained across scales of time and space. The more deeply we understand this interconnected system, the better we can protect it—ensuring that Earth remains a haven of stability amid the ever-changing cosmos.