In the vast, silent theater of the atomic world, most elements follow predictable rhythms. The protons and neutrons in their nuclei hold together in well-known configurations, giving rise to the stable elements that make up the matter around us—oxygen we breathe, iron in our blood, aluminum in our bikes and soda cans. But beyond this comfortable neighborhood, there lies a realm of instability, where the rules begin to bend, sometimes even break. It is here, at the outermost edges of nuclear existence, that physicists are beginning to hear strange whispers.
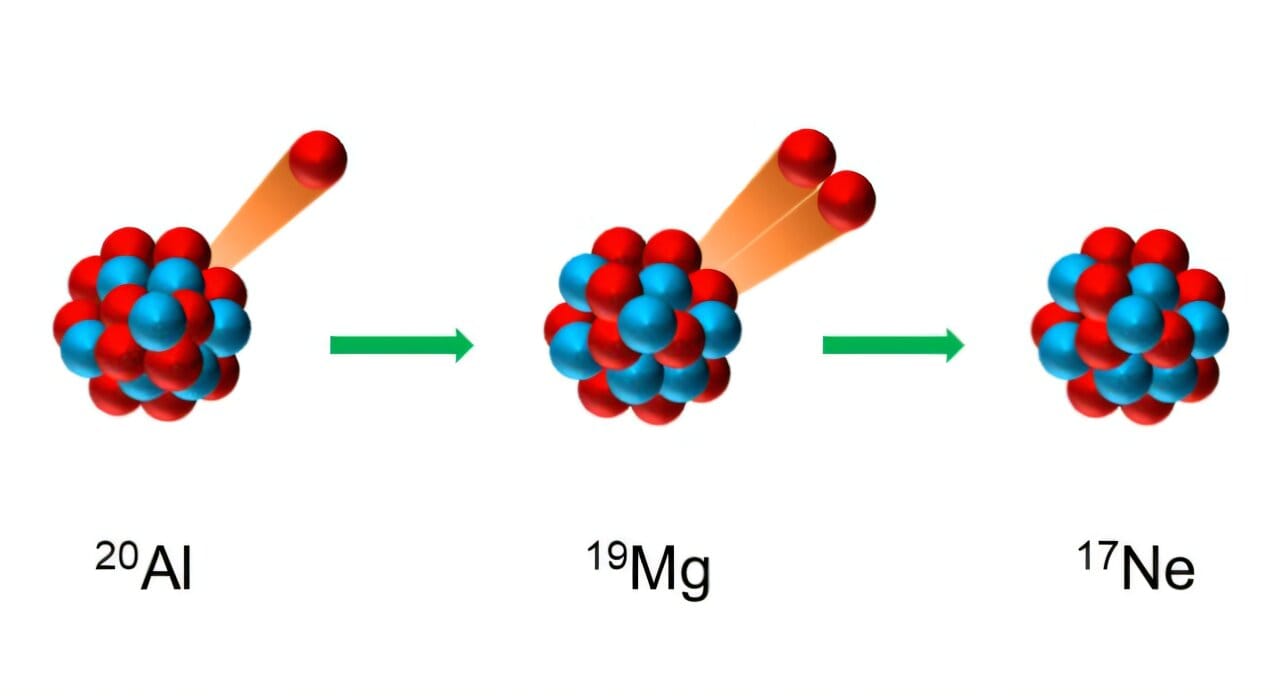
One such whisper has just been heard for the first time. It comes from a nucleus so exotic, so rare, and so fleeting that it had never been observed before: aluminum-20. This unstable isotope of aluminum decays in a way that borders on the unbelievable—it emits three protons in a cascade of nuclear fragmentation never before witnessed in this specific sequence.
A Discovery at the Limits of Matter
On July 10, a team of physicists from the Institute of Modern Physics (IMP) of the Chinese Academy of Sciences (CAS), working with international collaborators at the GSI Helmholtz Center for Heavy Ion Research in Germany, announced the first observation and spectroscopic study of aluminum-20. Their work, published in Physical Review Letters, does more than just add a name to the growing catalog of known isotopes—it marks a milestone in our quest to understand what holds the atomic nucleus together, and what finally tears it apart.
What makes aluminum-20 special is not just that it exists briefly before vanishing; it’s how it vanishes. The decay pathway it follows is one of the rarest ever seen. First, it emits a single proton, turning into magnesium-19. Then, that magnesium-19—already unstable—simultaneously ejects two more protons, collapsing into neon-17. It’s like watching a tightrope walker step forward and instantly split into three tumbling acrobats mid-air.
This process is known as three-proton emission, and aluminum-20 is the first nucleus ever discovered in which its direct decay product (magnesium-19) is itself a two-proton emitter. This extraordinary sequence provides nuclear physicists with a kind of Rosetta Stone—an intricate decay puzzle that reveals hidden information about nuclear forces, quantum behavior, and the boundaries of stability.
Beyond the Proton Drip Line
To understand why this discovery matters, it helps to understand where aluminum-20 sits on the nuclear chart. Nuclei are typically classified in terms of how many protons and neutrons they contain. The “valley of stability” is the heartland—where stable, naturally occurring isotopes live. Aluminum-27, for example, is stable and common in nature.
But aluminum-20? It lives far beyond the proton drip line, a kind of nuclear horizon. This is the point where adding more protons to a nucleus doesn’t lead to a stable atom, but instead causes the whole structure to spill over—like pouring water into a cup that can’t hold any more. Protons “drip” out, unable to be bound by the nuclear forces within.
Aluminum-20 has seven fewer neutrons than aluminum-27. It’s not just unstable; it’s fundamentally incapable of existing for more than a fleeting moment. Yet, in that moment, it provides a unique window into how nuclear matter behaves when pushed to its limits.
Associate Professor Xu Xiaodong of IMP, the study’s lead author, emphasized the importance of the finding: “This study advances our understanding of proton-emission phenomena, and provides insights into the structure and decay of nuclei beyond the proton drip line.”
Catching a Ghost in Motion
Capturing such an elusive nucleus required advanced technology and perfect timing. The experiment took place at the Fragment Separator of GSI in Darmstadt, Germany, using what is known as an in-flight decay technique. High-energy collisions smashed atomic nuclei apart, creating fleeting fragments like aluminum-20.
Once created, aluminum-20 began to decay immediately—faster than the blink of an eye. But the scientists didn’t need to see the nucleus directly. Instead, they measured the angular correlations of the particles it emitted—specifically, the three protons—using precision detectors. From these traces, like following footprints in the snow, they could reconstruct not only that aluminum-20 had existed, but how it had decayed.
Analyzing the angles at which the protons flew apart gave researchers clues about the sequence and timing of events, confirming that the decay occurred first as a one-proton emission, followed by a simultaneous two-proton emission from the daughter nucleus. It was not a random explosion but a structured unraveling—a kind of nuclear choreography.
Breaking Symmetry, Breaking Expectations
One of the most fascinating aspects of the study lies in what aluminum-20 didn’t do.
According to established principles in nuclear physics, particularly isospin symmetry, aluminum-20 and its mirror nucleus—neon-20, which has the same total number of nucleons but a different ratio of protons to neutrons—should have similar decay energies. But aluminum-20 didn’t follow the script. Its decay energy was significantly lower than predicted, suggesting a surprising symmetry breaking.
This deviation points to a deeper truth: nature, even at its most fundamental level, is full of exceptions. While isospin symmetry has served as a powerful guiding principle, the behavior of aluminum-20 hints at hidden complexities in the nuclear force, particularly when protons vastly outnumber neutrons. Theoretical calculations suggest that this discrepancy may be due to a difference in spin-parity—a quantum property that describes how particles behave under rotation—between aluminum-20 and its mirror partner.
Such deviations are not disappointments; they are gold mines for theorists. They tell us where our models fail, where the map ends and uncharted territory begins.
A Journey Through Nuclear History
To truly appreciate the significance of this discovery, one must zoom out and view it against the backdrop of nuclear physics history.
By the mid-20th century, scientists had cataloged common decay modes—alpha, beta, gamma radiation, and fission. These were the workhorses of nuclear transformation, and they remain essential for understanding natural radioactivity, nuclear energy, and medical imaging.
But in the 1970s, the story took a turn. Physicists observed single-proton radioactivity in certain rare isotopes. In the early 2000s, the discovery of two-proton radioactivity opened a new chapter. And now, in the 2020s, we are watching the first pages being written on three-proton emission—an exotic and almost poetic unraveling of matter at its most fundamental scale.
The ability to even observe such processes is a testament to the advances in accelerator technology, detector sensitivity, and computational modeling. It is also a testament to international collaboration—this study involved scientists from IMP, GSI, Fudan University, and over a dozen institutions worldwide. Together, they are slowly mapping the mysterious outskirts of the nuclear landscape.
Why It Matters
At first glance, studying a ghostly aluminum isotope that vanishes in microseconds may seem like academic curiosity. But such work gets at the core questions of existence.
What are the limits of matter? How do atomic nuclei hold together, and why do they sometimes fall apart? What strange rules govern the behavior of protons and neutrons when pushed to extremes?
Answering these questions has far-reaching implications—from understanding the elements forged in supernovae to predicting the behavior of radioactive materials used in medicine and energy. Moreover, every new isotope discovered, every unusual decay mapped, sharpens our theories of quantum mechanics and nuclear force.
And sometimes, discoveries like aluminum-20 teach us something even deeper: that even the most fundamental building blocks of the universe still hold secrets. Secrets that we can only uncover by daring to look beyond what is stable, safe, and known.
From the Edge, a New Frontier
As of today, scientists have discovered more than 3,300 nuclides—yet fewer than 300 are stable. The rest are living in borrowed time, fated to decay, often in astonishing ways. Aluminum-20 now joins that list, a name etched into the annals of nuclear physics not for its longevity, but for its fleeting brilliance.
The three protons it left behind are more than particles. They are breadcrumbs along the path toward a deeper understanding of the forces that knit the cosmos together. In their delicate angular dance, we glimpse the universe whispering one of its many secrets.
And the next time we look at the periodic table, perhaps we will remember that behind each square lies not just an element, but a story—some of which, like aluminum-20, are only beginning to be told.
Reference: X.-D. Xu et al, Isospin Symmetry Breaking Disclosed in the Decay of Three-Proton Emitter Al20, Physical Review Letters (2025). DOI: 10.1103/hkmy-yfdk