Every breath you take, every car engine that roars, every lightning bolt across the sky, and every steaming cup of coffee cooling beside you—each of these moments is governed by a powerful, universal set of principles known as thermodynamics. This field of physics describes the flow of energy in the universe. It might sound like an abstract or dry concept, but in truth, thermodynamics shapes everything from the birth of stars to the metabolism in your cells.
At its heart, thermodynamics is the science of energy, heat, and work. It investigates how energy moves, transforms, and degrades. Whether you’re dealing with boiling water or building a spacecraft, thermodynamics holds the key to understanding how systems behave. It tells us why machines need fuel, why no engine can be 100% efficient, and even why time seems to move in one direction.
This article will take you on a fascinating journey through the basics of thermodynamics. You’ll discover its foundational laws, how they influence everything from refrigerators to rockets, and how these laws reveal deep truths about the nature of our universe.
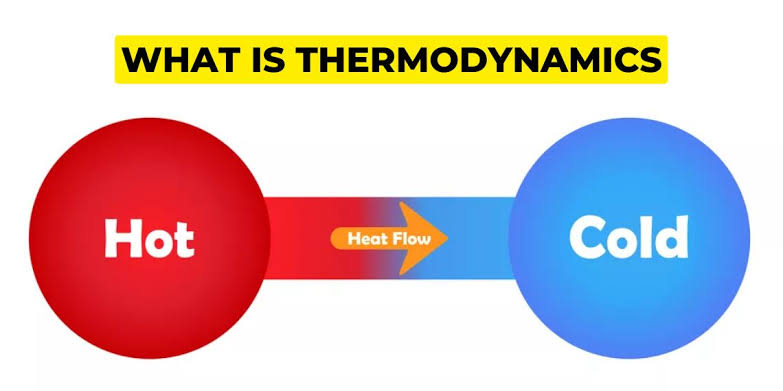
What is Thermodynamics?
The word “thermodynamics” originates from the Greek words “therme,” meaning heat, and “dynamis,” meaning power. Together, they express the idea of how heat produces motion or work—a foundational concept in the 19th century as steam engines began powering the industrial revolution.
But thermodynamics has grown far beyond steam and pistons. Today, it is a universal science that applies to chemistry, biology, meteorology, astrophysics, engineering, and even information theory. At its core, thermodynamics is concerned with energy—how it flows, how it changes form, and how it affects matter.
A thermodynamic system is the part of the universe we’re focusing on. Everything outside of it is called the surroundings. The boundary between the system and its surroundings can be real (like the walls of a container) or imaginary. There are different kinds of systems: open (exchanges both matter and energy), closed (exchanges only energy), and isolated (exchanges nothing).
Understanding thermodynamics means understanding how these systems evolve and behave under various conditions. And that’s where the laws of thermodynamics come in.
The Zeroth Law: The Foundation of Temperature
Before the first law could even be articulated, scientists realized they needed a consistent way to define and measure temperature. That’s where the Zeroth Law of Thermodynamics comes in.
The Zeroth Law states:
If system A is in thermal equilibrium with system B, and system B is in thermal equilibrium with system C, then A is also in thermal equilibrium with C.
This might seem obvious, but it’s crucial because it allows us to define temperature in a meaningful way. If two systems are in thermal equilibrium, they must have the same temperature. This law allows the use of thermometers. When your thermometer reaches the same temperature as your body, you can infer your body’s temperature based on the thermometer’s reading.
In essence, the Zeroth Law underpins the very concept of temperature and lets us use it as a measurable quantity in science and everyday life.
The First Law: Conservation of Energy
The First Law of Thermodynamics is one of the most fundamental principles in all of physics. It is essentially a restatement of the law of conservation of energy, tailored for thermal systems.
The First Law states:
Energy cannot be created or destroyed; it can only be transferred or converted from one form to another.
In mathematical terms, the law is often expressed as:
ΔU = Q – W
Where:
- ΔU is the change in the internal energy of the system
- Q is the heat added to the system
- W is the work done by the system
Imagine you’re heating a gas in a piston. The heat (Q) goes into the system, increasing its internal energy (U) and allowing it to expand and do work (W) by pushing the piston. Energy isn’t lost or gained—it just changes form.
This law gives rise to the idea of internal energy: the energy stored in a system due to the kinetic and potential energy of its molecules. It also provides the foundation for understanding how engines work, how chemical reactions produce energy, and how living organisms fuel their biological processes.
One of the most profound implications of the First Law is that there’s no such thing as a free lunch when it comes to energy. You can’t get more energy out of a system than you put in. Perpetual motion machines of the first kind—devices that produce more energy than they consume—are impossible.
The Second Law: The Arrow of Time and the Rise of Entropy
While the First Law tells us that energy is conserved, the Second Law of Thermodynamics tells us that not all energy transfers are equally useful. This law introduces a new and crucial concept: entropy.
The Second Law states:
In any natural thermodynamic process, the total entropy of the system and its surroundings always increases.
Entropy is often described as a measure of disorder or randomness. But more precisely, it quantifies the number of ways a system can be arranged microscopically while still appearing the same macroscopically. Higher entropy means more disorder, more possibilities, and less usable energy.
This law explains why energy tends to spread out and why systems naturally move from order to disorder. A hot cup of coffee left on a table cools down—it never spontaneously heats up. A broken glass does not reassemble itself. These are everyday manifestations of the Second Law.
Entropy also gives us the arrow of time—a direction in which time flows. While the laws of mechanics are reversible, entropy only increases with time, giving time its irreversible quality.
This law has profound consequences in all areas of science. In engines, it limits efficiency—some energy is always lost as waste heat. In biology, it drives the flow of energy through ecosystems and the breakdown of food into usable molecules. In cosmology, it helps explain the eventual fate of the universe: a state of maximum entropy known as heat death.
The Third Law: The Unattainable Absolute Zero
The Third Law of Thermodynamics deals with the behavior of systems as they approach absolute zero, the lowest possible temperature at which all molecular motion ceases.
The Third Law states:
As a system approaches absolute zero, the entropy of a perfect crystal approaches zero.
Absolute zero, or 0 Kelvin (-273.15°C), is the theoretical point where atoms stop moving entirely. While we can get very close, it is impossible to reach absolute zero in practice. The closer we get, the harder it becomes to remove that last bit of energy.
This law is essential for understanding the limits of cooling technologies and explains why superconductors and superfluids—materials with bizarre and exotic behaviors—emerge at temperatures near absolute zero. It also supports the idea that there is a fundamental minimum to the disorder a system can have.
Thermodynamic Processes and Cycles
Thermodynamic systems undergo various processes—transformations that change the system’s pressure, volume, temperature, and internal energy. These include:
- Isothermal processes (constant temperature): Heat flows in or out of the system while temperature remains steady.
- Adiabatic processes (no heat exchange): Changes in internal energy result entirely from work done.
- Isobaric processes (constant pressure): Heat exchange changes volume and temperature.
- Isochoric processes (constant volume): Heat changes internal energy and pressure.
These processes form the basis of thermodynamic cycles, which are sequences of processes that return a system to its original state. The most famous is the Carnot cycle, which represents the maximum possible efficiency any heat engine can achieve. Real-world engines, like those in cars or power plants, are often based on cycles like the Otto or Rankine cycles.
Understanding these cycles allows engineers to design better engines, improve energy efficiency, and minimize waste. Thermodynamic cycles are also crucial in refrigeration, air conditioning, and power generation.
Applications of Thermodynamics in Everyday Life
The principles of thermodynamics are not just theoretical—they affect nearly every aspect of our lives. Here are some key areas where they shine.
Heat Engines and Transportation
Cars, trucks, trains, and airplanes rely on engines that convert chemical energy in fuel into mechanical work. Thermodynamics governs every step of that conversion. Combustion increases temperature and pressure, pushing pistons or turbines and producing motion. The Second Law limits how much of the energy can actually be converted into useful work.
Refrigerators and Air Conditioners
These devices use thermodynamic cycles in reverse. Instead of turning heat into work, they use work (electricity) to move heat from a colder area to a warmer one—essentially fighting entropy by removing heat from your food or your living room.
Power Plants
Electricity generation in coal, nuclear, and gas power plants involves heating water to produce steam, which spins turbines. Again, thermodynamic cycles determine efficiency and guide design improvements to reduce emissions and fuel use.
Biological Systems
Living organisms are exquisite examples of thermodynamic systems. Our cells extract energy from nutrients through metabolic pathways governed by enzymes. ATP, the cellular “energy currency,” is produced and used based on thermodynamic gradients. Even processes like respiration and photosynthesis follow the laws of energy and entropy.
Climate and Weather
The Earth’s climate system is driven by the uneven heating of the planet’s surface by the Sun. Thermodynamics explains how heat moves through the atmosphere and oceans, how storms form, and why weather patterns develop as they do. Climate models depend heavily on thermodynamic equations.
Chemical Reactions and Industry
Chemical engineering relies on thermodynamics to design reactors, manage energy flow, and control the outcomes of reactions. Whether synthesizing pharmaceuticals or refining oil, thermodynamic principles ensure reactions proceed efficiently and safely.
Information Theory and Thermodynamics
An intriguing connection has emerged between thermodynamics and information theory, thanks to the work of scientists like Claude Shannon and Rolf Landauer. Landauer’s Principle states that erasing one bit of information in a computer requires a minimum amount of energy and generates entropy.
This connection has profound implications for the future of computing, especially as we move toward smaller, faster, and more energy-efficient devices. It also ties the physical world to the abstract realm of data, suggesting that information itself is a physical quantity governed by thermodynamic laws.
The Future: Thermodynamics in Emerging Technologies
As humanity pushes the boundaries of technology, thermodynamics remains a critical guide. In nanotechnology, for example, tiny machines operate under conditions where thermal fluctuations dominate, requiring a deep understanding of energy at small scales.
In space exploration, thermodynamics governs life-support systems, propulsion, and energy generation. It also plays a role in developing new materials, optimizing energy storage, and reducing greenhouse gas emissions.
Advances in quantum thermodynamics are beginning to explore how thermodynamic laws apply at the level of individual atoms and particles—pushing the boundaries of what we thought we knew.
Conclusion: The Science That Powers the Universe
Thermodynamics is more than a set of equations or abstract principles—it is a profound description of how the universe functions. From the smallest cell to the largest galaxy, from steam engines to solar panels, from entropy to energy conservation, thermodynamics governs change itself.
Understanding its laws opens the door to innovation, sustainability, and a deeper appreciation for the natural world. Whether you’re an engineer, a scientist, a student, or simply a curious mind, mastering the basics of thermodynamics means grasping the rules that govern life, technology, and the cosmos.
In a universe constantly moving toward disorder, thermodynamics helps us find the patterns, harness the energy, and shape the future.