Among the countless elements that thread through the fabric of the universe, phosphorus holds a unique and intimate connection to life. On Earth, phosphorus is woven into DNA and RNA, the very molecules that carry the blueprint of life. It fuels our cells in the form of adenosine triphosphate (ATP), the energy currency of biology. Without phosphorus, life as we know it would collapse into silence.
Yet this element has a darker, more volatile side. When bonded with hydrogen, it forms phosphine (PH₃), a molecule infamous for its toxicity and explosive nature. On our planet, phosphine is not something you would want to inhale—it is deadly in even small doses. But its appearance in the skies of Jupiter and Saturn has given it a strange, double life: both a poison and a potential signpost for biology. In the realm of astrobiology, phosphine is regarded as a possible biosignature gas, one of the chemical whispers that could hint at the presence of life elsewhere.
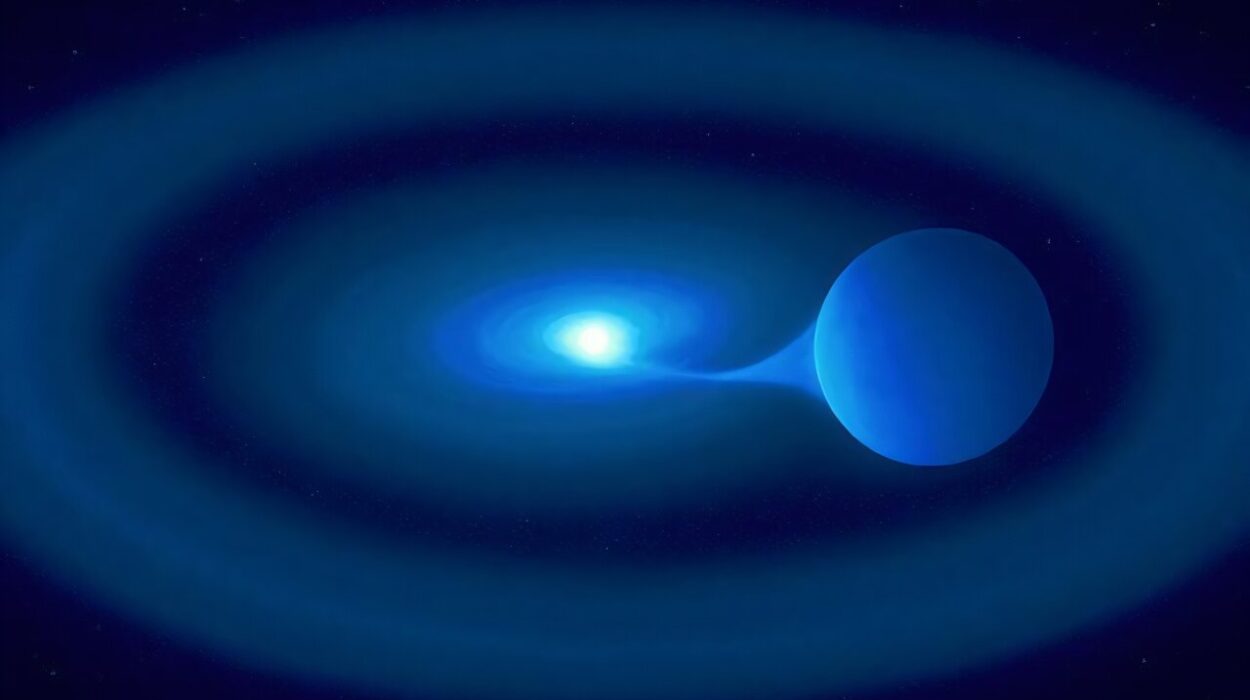
And now, phosphine has appeared in an unexpected place: the atmosphere of a cool, ancient brown dwarf named Wolf 1130C.
A Discovery Beyond Expectation
Wolf 1130C is not a planet. It is what astronomers call a brown dwarf—an object too massive to be a planet, yet too small to ignite like a star. Often described as “failed stars,” brown dwarfs occupy the twilight zone of the cosmos. They glow faintly, not from nuclear fusion like the Sun, but from residual heat left over from their formation.
For years, astronomers have scoured the skies for signs of phosphine in brown dwarfs and giant exoplanets. Theoretically, the molecule should be there. The hydrogen-rich atmospheres of these worlds ought to allow phosphine to form naturally, just as it does on Jupiter and Saturn. Yet observation after observation came back empty, challenging our models of atmospheric chemistry.
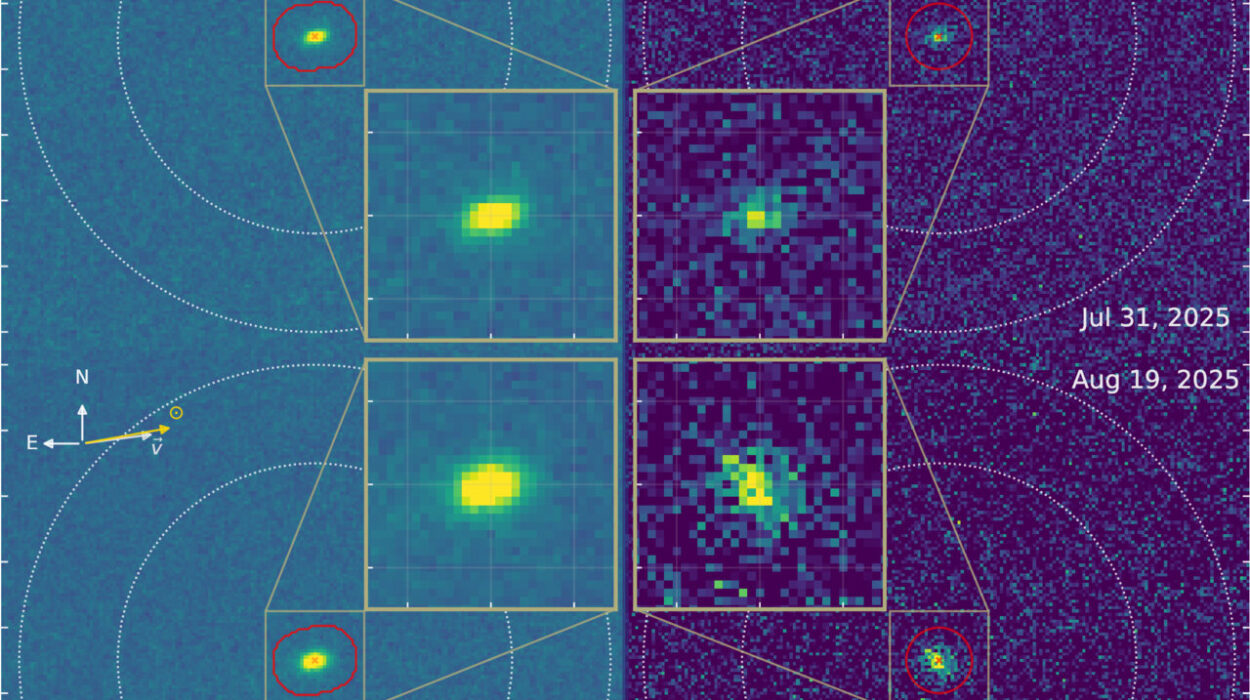
That changed with the keen eye of the James Webb Space Telescope (JWST). For the first time, researchers led by Adam Burgasser of the University of California San Diego detected phosphine in Wolf 1130C’s atmosphere. Using the telescope’s sensitive infrared instruments, they uncovered spectral fingerprints of the elusive molecule.
The surprise was not simply its presence, but the question it raised: why does this ancient brown dwarf have phosphine when so many others do not?
The Puzzles of Chemistry
In science, every answer births new questions. The presence of phosphine in Wolf 1130C forces astronomers to confront a mystery: if theory predicts phosphine should be common in such atmospheres, why do we only see it here?
One possible clue lies in Wolf 1130C’s unique composition. Unlike many stars and brown dwarfs, it is metal-poor—meaning it has fewer elements heavier than hydrogen and helium. In astrophysics, “metals” include not just iron or copper, but anything beyond helium. The Sun, for example, is relatively rich in these elements compared to Wolf 1130C.
In a metal-rich environment, phosphorus may bind with oxygen to form other compounds such as phosphorus oxides, preventing phosphine from accumulating. But in the stripped-down chemistry of Wolf 1130C, oxygen may be scarce enough that phosphorus is left free to combine with hydrogen, producing phosphine in measurable amounts.
The implication is profound. The chemistry of planets and stars may depend far more strongly on metallicity than we realized. Phosphorus, an element so critical for life on Earth, may take on different guises depending on the chemical canvas of its environment.
A Star System’s Legacy
Wolf 1130C does not exist alone. It orbits a pair of stellar companions: a cool red star, Wolf 1130A, and a dense white dwarf, Wolf 1130B. Together, the three form a strange celestial family bound by gravity.
The presence of a white dwarf introduces another possible explanation for the phosphine. White dwarfs are the remnants of stars that have exhausted their fuel and shed their outer layers. Occasionally, these compact objects flare back to life in violent bursts called novae—explosions triggered when the star accumulates material and undergoes runaway nuclear reactions.
Such outbursts can seed surrounding space with heavy elements, including phosphorus. If Wolf 1130B experienced such events in the past, it may have polluted its neighborhood with phosphorus, leaving behind a chemical legacy now detectable in Wolf 1130C’s skies. Though astronomers have not witnessed such an event in this system in recorded history, these outbursts can occur over timescales far longer than human observation. The traces of ancient stellar violence may linger still.
The Work Behind the Discovery
The path from raw telescope data to scientific insight is long and intricate. JWST provided the light, but it took sophisticated modeling to translate spectral lines into chemical abundances.
Eileen Gonzales, an assistant professor of astronomy at San Francisco State University, employed a method called atmospheric retrievals to analyze the data. By comparing observed spectra to models of possible atmospheric compositions, she essentially reverse-engineered the recipe of Wolf 1130C’s atmosphere.
Her results revealed phosphine at abundances close to what theory had long predicted: about 100 parts per billion. That may sound vanishingly small, but in the vast envelope of a brown dwarf, it is enough to stand out unmistakably. For astronomers who had spent years chasing a molecule that never appeared, the confirmation was exhilarating.
Why This Matters for Life
Phosphine is not just another exotic molecule. On Earth, it is associated with anaerobic processes—life that thrives without oxygen. Microbes in oxygen-deprived environments such as swamps and marshes produce trace amounts of phosphine, which then escapes into the atmosphere. Because non-biological sources of phosphine are so limited on rocky planets, its detection elsewhere has been championed as a possible sign of alien life.
The detection of phosphine on Wolf 1130C, however, provides a cautionary tale. Here, in a world where life is not expected, phosphine has appeared through purely chemical means. That does not invalidate its potential as a biosignature, but it complicates the story. To use phosphine as a reliable indicator of life, we must first understand all the non-biological pathways by which it can form. Brown dwarfs like Wolf 1130C, lifeless laboratories in space, become essential to this endeavor.
As Adam Burgasser explains, studying phosphine in such environments is not a distraction from the search for life—it is a foundation for it. Only by untangling the chemical processes that produce phosphine in dead worlds can we recognize when it might truly signal the presence of biology on living ones.
A Window into the Galaxy’s Chemistry
Beyond astrobiology, the discovery speaks to larger questions about the Milky Way. Phosphorus itself is rare compared to other life-essential elements such as carbon or nitrogen. Understanding where and how it is made, distributed, and transformed across the galaxy is critical for tracing the ingredients of habitability.
If novae are major sources of phosphorus, as some studies suggest, then white dwarfs like Wolf 1130B may have played an outsized role in seeding the galaxy with this vital element. The discovery of phosphine in Wolf 1130C could therefore illuminate not just the chemistry of one brown dwarf, but the chemical history of the entire galaxy.
Every molecule detected tells a story: of ancient stars that lived and died, of planetary atmospheres sculpted by their environment, of the cosmic alchemy that makes life possible. Phosphine’s tale is still being written, and Wolf 1130C is now one of its unexpected narrators.
The Road Ahead
This discovery is only a beginning. The JWST has opened a new era of precision astronomy, and Wolf 1130C will not be the last brown dwarf probed for phosphine. Researchers now plan to survey other metal-poor brown dwarfs to test whether the same conditions favor phosphine formation. Each detection—or absence—will refine our understanding of how phosphorus behaves under alien skies.
The answers may ripple far beyond the study of brown dwarfs. They will inform our models of giant exoplanets, enrich our understanding of the galaxy’s chemistry, and sharpen the tools we use in the search for life. The next time phosphine appears in the atmosphere of a rocky planet, we will be better equipped to ask: is this chemistry, or is this biology?
A Molecule That Connects Us
At its core, the story of phosphine is a story of connection. From swamps on Earth to the stormy clouds of Jupiter, from the toxic skies of a brown dwarf to the quest for extraterrestrial life, this simple molecule binds together diverse realms of science. It is a reminder that chemistry does not stop at the boundaries of planets, nor at the limits of our imagination.
Phosphorus was born in the hearts of stars, scattered across space in supernovae and novae, gathered into worlds like ours, and woven into the fabric of biology. Now, through the lens of the James Webb Space Telescope, we glimpse its transformations in places far beyond Earth.
In every detection lies both mystery and meaning. Phosphine challenges us to understand the universe more deeply, to recognize the subtle interplay of chemistry and environment, and to approach the search for life with both caution and hope.
Wolf 1130C, a quiet brown dwarf circling its stellar companions 54 light-years away, has added a new chapter to this unfolding saga. It reminds us that even in the most unexpected corners of the cosmos, elements essential to life are at play—sometimes hidden, sometimes revealed, always waiting for us to notice.
And so the question continues, echoing through the night sky: in the phosphine of distant worlds, are we witnessing mere chemistry, or the faintest whispers of life itself?
More information: Adam J. Burgasser et al, Observation of undepleted phosphine in the atmosphere of a low-temperature brown dwarf, Science (2025). DOI: 10.1126/science.adu0401. www.science.org/doi/10.1126/science.adu0401