The human brain is often described as the most complex object in the known universe. With nearly 86 billion neurons firing in intricate patterns and networks, it is both the engine of our thoughts and the mystery that defines us. For decades, neuroscientists have sought to understand how specific brain regions and circuits give rise to memory, emotion, perception, and movement. To do this, they rely on advanced imaging techniques and neural probes—tiny electronic devices that can record the electrical impulses, or “spikes,” that neurons produce.
Traditional probes have already revealed fascinating insights, from how neurons in the visual cortex detect edges to how networks of cells generate movement. Yet these tools have their limits. Most existing devices are flat, two-dimensional (2D) structures that cannot fully match the brain’s inherently three-dimensional (3D) architecture. This mismatch has restricted the depth and detail of our recordings, leaving gaps in our ability to map the brain’s living symphony of signals.
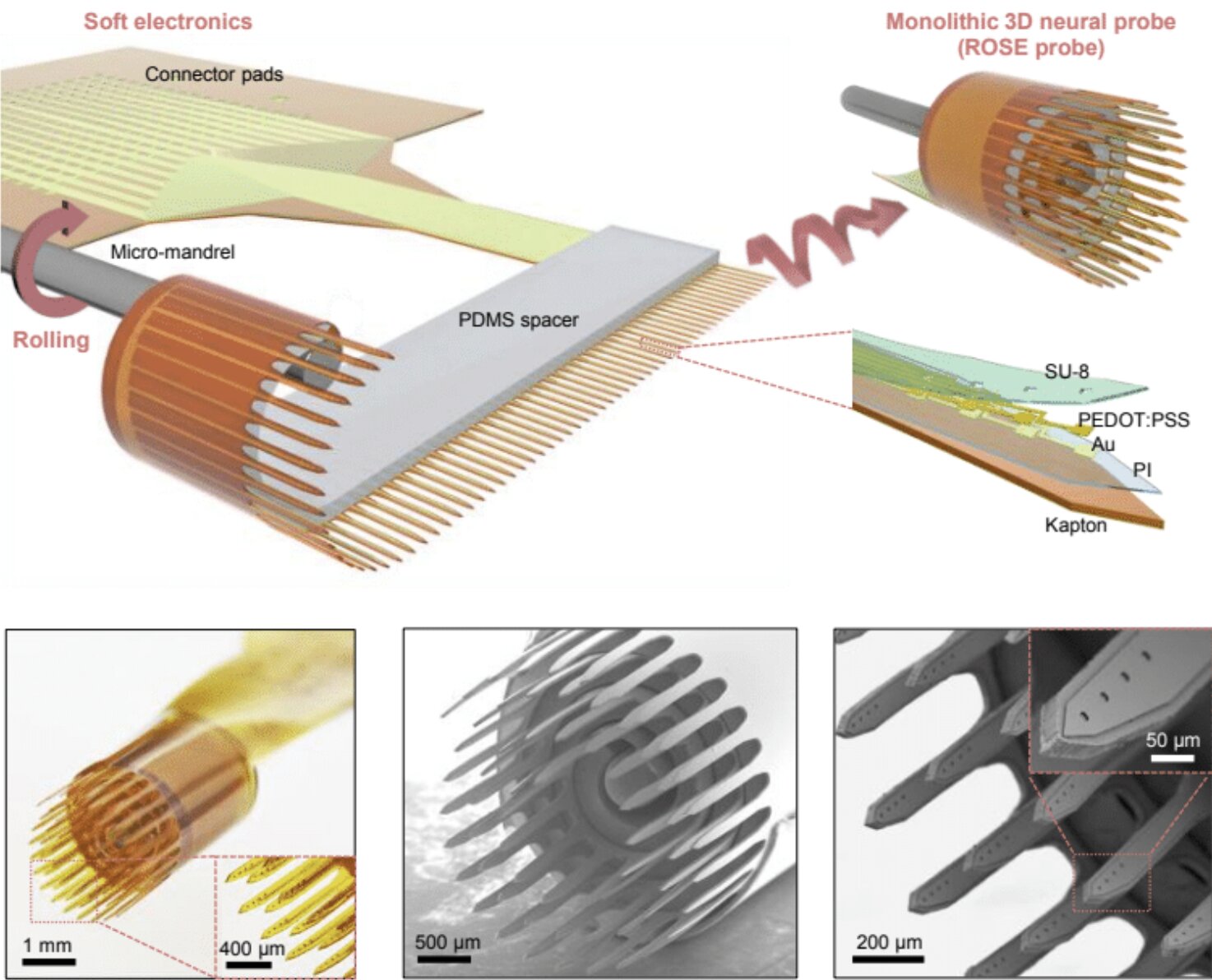
Now, a team of researchers from Dartmouth College, the University of Pittsburgh, Oklahoma State University, and other institutions has developed a revolutionary new approach: rolling flexible electronic devices into soft, cylindrical structures that more naturally align with the brain’s 3D networks. Their invention—called ROSE probes (short for rolling of soft electronics)—promises to open entirely new possibilities in neuroscience and medicine.
Inspired by a Swiss Roll
The breakthrough idea came from what the team calls the “dimensional mismatch.” Conventional probes are flat, rigid devices built using the same methods employed in semiconductor manufacturing. The brain, on the other hand, is soft, flexible, and three-dimensional.
Hui Fang, senior author of the study published in Nature Electronics, recalls the moment of inspiration: “One day it occurred to us that, because we’re working in this flexible electronics world, why not take advantage of the softness of things? If we roll things up, we can realize 3D probes at the scale of an organ like the brain.”
The analogy the researchers use is both vivid and approachable: think of a Swiss roll cake. When the flat layers of sponge and cream are rolled, they form a spiral with many protruding edges. Similarly, when the team rolled up their flexible electronic sheets, they created cylindrical probes with numerous thin “shanks” extending outward. Each shank carries multiple electrodes, spaced apart by soft materials that maintain their distance from each other.
This clever design allows hundreds of electrodes to be embedded in a single probe, far surpassing the capacity of today’s gold-standard devices like the Utah Array, which typically places just one electrode at each tip. The result is not only a higher density of recording sites but also the ability to capture activity across different depths within the brain’s intricate circuitry.
Recording the Symphony of Neurons
Why does this matter? Because neurons are incredibly small, yet densely packed into specialized regions of the brain. To understand how they communicate, scientists need to record not just one or two signals, but hundreds or even thousands at once—ideally in three dimensions.
The ROSE probes make this possible. By adjusting parameters such as the spacing between shanks, the thickness of the spacer, and the number of electrodes per shank, researchers can customize the probes to target specific brain regions or functions.
In tests with awake mice and rats, the team demonstrated that their 3D probes could record more distributed and detailed activity than traditional devices. They revealed, for instance, new insights into orientation tuning—how neurons respond to the direction of visual stimuli. This higher-resolution recording enables more accurate decoding of brain signals, bringing us closer to understanding how groups of neurons cooperate to generate perception and behavior.
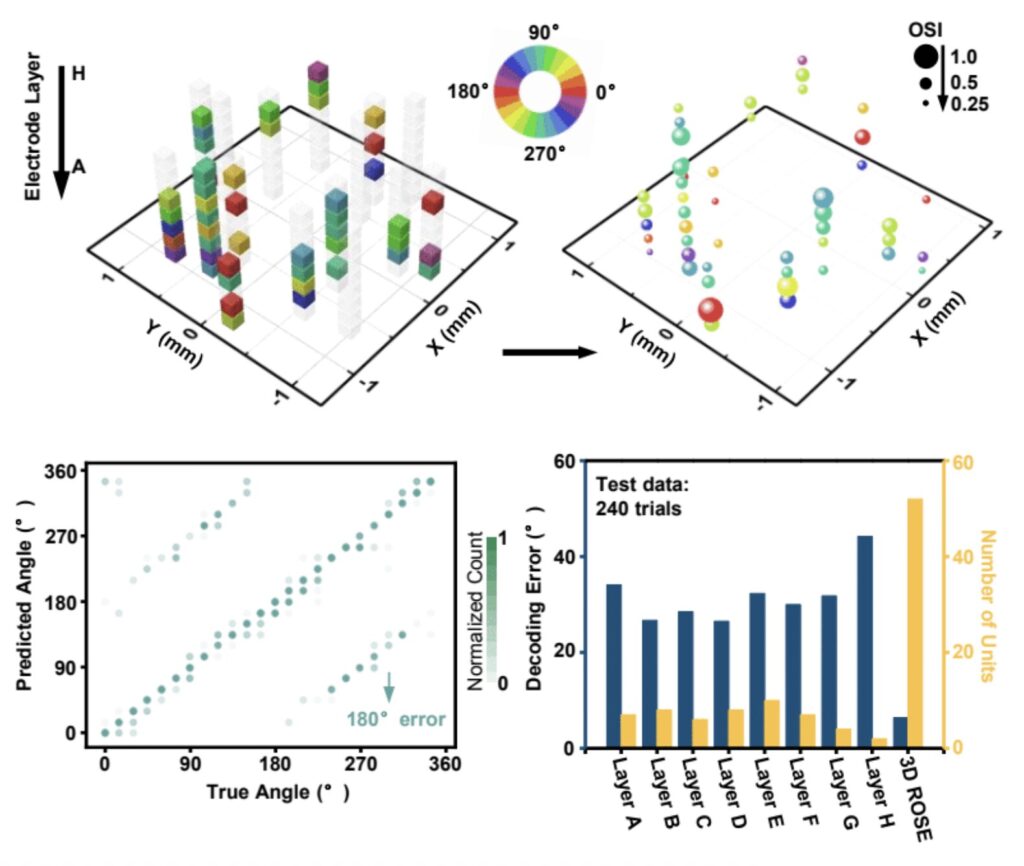
Equally important, the soft design of the ROSE probes reduces tissue stress and inflammation compared to stiff silicon-based probes. This means they can potentially remain implanted for longer periods, capturing data across weeks or months—a critical advantage for both research and medical applications.
From Lab to Clinic: Transformative Potential
The possibilities of this technology extend far beyond basic neuroscience. By providing a richer, more detailed window into brain activity, ROSE probes could accelerate progress in treating neurological disorders and developing brain–machine interfaces.
One potential application is in motor prosthetics. For individuals who have lost the ability to move due to spinal cord injuries or other conditions, these devices could translate brain signals into commands for robotic limbs or computer cursors. With more precise recordings from larger populations of neurons, the prosthetics could become faster, more intuitive, and more responsive to the user’s intentions.
Another emerging use is in visual neuro-prosthetics, or so-called “bionic eyes.” By interfacing with the brain’s visual pathways, these systems aim to restore partial vision to people with blindness or severe visual impairments. The dense, customizable electrodes of the ROSE probes could make these devices more effective, providing higher resolution and more natural visual experiences.
The probes could also support fundamental research into conditions such as epilepsy, Parkinson’s disease, and depression, where abnormal neural activity plays a central role. By mapping the brain’s activity in greater detail, scientists may identify new targets for therapies or refine deep brain stimulation treatments already in use.
The Road Ahead
While the early results are promising, the journey is far from over. The team is now working to improve the chronic biocompatibility of their probes—ensuring that they can safely remain in the brain for months or even years without losing effectiveness or causing harm. As Fang explains, “This will open a lot of opportunities from fundamental neuroscience to clinical applications. Second, we’d like to pursue neuro-prosthetic studies and human translation.”
If successful, the technology could become a cornerstone of next-generation neuroscience and medicine. Just as the microscope transformed biology centuries ago, 3D neural probes like ROSE may redefine how we explore, understand, and heal the human brain.
The Emotional Dimension of Discovery
At its heart, this breakthrough is about more than just technology—it’s about human curiosity and compassion. Neuroscientists are not merely chasing abstract knowledge; they are striving to unlock the secrets of memory, perception, and consciousness. Medical researchers are searching for ways to restore movement to those paralyzed, or sight to those who live in darkness.
The ROSE probes remind us that innovation often comes from daring to see problems differently. By rolling up a flat device, these researchers have opened a new dimension of possibility—one that mirrors the layered, spiraled complexity of the brain itself.
The story of these 3D neural probes is not just about electrodes and experiments; it is about hope. Hope that one day, technology will bridge the gap between damaged neurons and lost abilities. Hope that by understanding the brain more deeply, we will understand ourselves more fully.
And perhaps most of all, hope that science, guided by both precision and imagination, can continue to transform the lives of people around the world.
More information: Yi Qiang et al, Monolithic three-dimensional neural probes from deterministic rolling of soft electronics, Nature Electronics (2025). DOI: 10.1038/s41928-025-01431-0.