In an exciting breakthrough, a team of researchers, including theorists from UC San Diego, has demonstrated for the first time that heat transfer in the form of infrared (IR) radiation can play a more significant role in influencing chemical reactions than traditional methods such as convection and conduction. This innovative research, conducted in both experimental and theoretical domains, shows the potential for harnessing infrared radiation to control chemical processes with unprecedented precision.
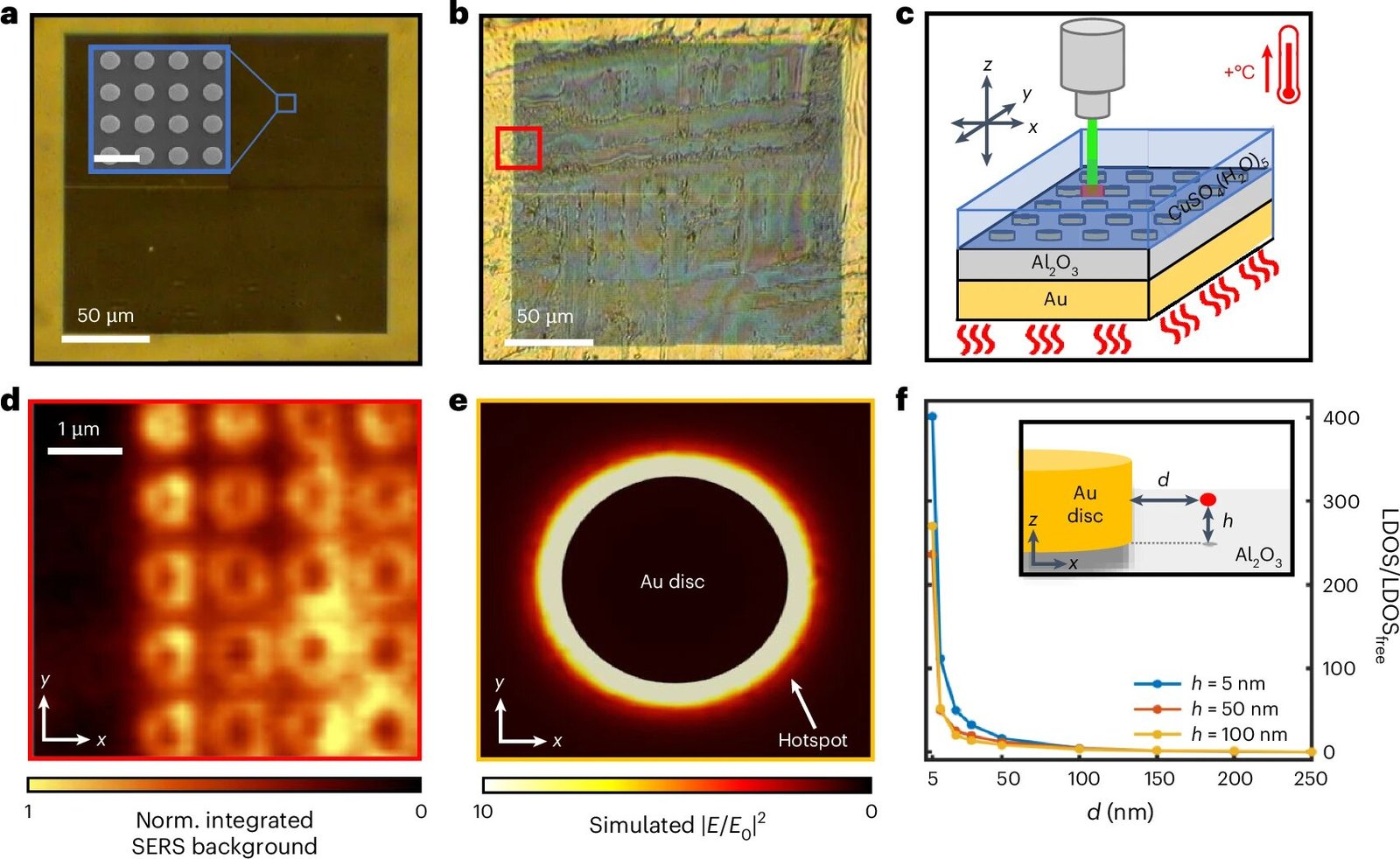
The team’s findings, published in the prestigious Nature Chemistry journal, center around an unexpected mechanism of heat transfer that could lead to new approaches in catalytic systems and optoelectronic processes. The research shows that by confining infrared light waves in an optical cavity, researchers can influence the thermal dehydration of an inorganic crystal, copper sulfate pentahydrate, using light rather than heat in the conventional sense. This offers a fresh perspective on how heat transfer mechanisms may be used to modify the way chemical reactions proceed at the molecular level.
Investigating the Role of Infrared Radiation
The researchers focused on the dehydration process of copper sulfate pentahydrate (CuSO₄·5H₂O), a well-known inorganic compound often used in chemical education and industrial applications. Typically, dehydration of such crystals involves heating the material to drive off the water molecules trapped in the crystalline structure. In traditional heat transfer methods, this is accomplished through convection, where heat flows via the movement of fluid or gases, and conduction, where heat is transferred directly from one substance to another through direct contact.
However, the team found that infrared radiation—particularly within the wavelength region specific to the absorption features of the material—could accelerate the dehydration reaction at lower temperatures than previously possible. More specifically, the research demonstrated that light-matter interactions in the form of vibrational coupling led to the formation of polaritons—hybrid states that emerge when light and matter interact strongly.
These polaritons enabled faster heat absorption by the copper sulfate pentahydrate, and as a result, dehydration occurred at temperatures as much as 14°C lower than the typical threshold. This effect indicates that radiative energy transport, wherein photons transfer heat between regions without the need for direct contact, plays a previously overlooked role in thermal processes.
Polaritons and Light-Matter Interaction
Central to this discovery is the concept of polaritons, which arise when electromagnetic radiation, in this case infrared light, interacts with the vibrational modes of matter. Polaritons are hybrid particles that exhibit both light and matter properties. In the experiment, infrared photons generated within the optical cavity coupled with the vibrational states of the copper sulfate pentahydrate crystal, leading to a scenario where the system’s internal energy could be more efficiently transferred and dissipated, lowering the necessary temperature for the reaction.
This process had previously been underestimated in thermochemical research. In classical heat conduction, energy moves from a hot object to a cooler one until thermal equilibrium is reached. However, with the involvement of polaritons and IR radiation, the heat transfer mechanism extends beyond simple thermal diffusion, offering a new perspective for controlling chemical reactions through non-contact methods.
Implications for Catalysis and Optoelectronics
The broader implications of this discovery stretch far beyond the thermal dehydration of inorganic compounds. Researchers suggest that this technique could be used to modify thermochemical processes, opening the door to more energy-efficient ways of conducting reactions, especially those that involve temperature-sensitive materials or require fine control over the reaction rate.
Moreover, the ability to manipulate thermal reactions through light interactions could also significantly impact the development of catalytic systems. Catalysts—substances that speed up chemical reactions—could benefit from tailored infrared radiation sources, providing precise control over reaction dynamics without the need for traditional heating. This could make reactions more efficient, allowing them to proceed at lower temperatures or in more controlled environments, saving both energy and time.
The use of optical cavities to manipulate the way energy is transferred at the atomic and molecular levels is also poised to have significant ramifications for the field of optoelectronics. Devices such as lasers, photo detectors, and solar cells that rely on the interaction between light and matter could experience new advancements if they can exploit these light-matter hybrid states, creating more efficient and cost-effective devices for energy conversion, information transmission, and sensor technology.
Paving the Way for Future Research
This groundbreaking work offers a fresh route to understanding how light can influence chemical dynamics, allowing researchers to explore the intricate relationship between radiation, temperature, and reaction rates in novel ways. Optical cavities, where light is confined and manipulated, represent a powerful tool in this exploration, and this approach could one day be integrated with other technologies in the design of next-generation catalysts, reactors, and even materials for use in quantum computing and nanotechnology.
Furthermore, as researchers continue to study the interaction between light and matter at increasingly fine scales, future studies may reveal additional ways in which infrared radiation can be used to control complex chemical reactions in various environments, ranging from environmental science to pharmaceuticals.
Conclusion
The joint experimental-theoretical work conducted by UC San Diego and its collaborators has proven that infrared radiation can surpass traditional thermal conduction and convection in modifying chemical reactions. By using optical cavities to manipulate light-matter interactions, the researchers have introduced a novel method of influencing thermochemical processes, demonstrating that infrared radiation can lower the required temperature for reactions, as shown by the thermal dehydration of copper sulfate pentahydrate. This method could revolutionize catalysis, chemical processing, and optoelectronics, enabling greater precision, energy efficiency, and innovative applications for industrial and scientific use. The work, which pushes the boundaries of heat transfer science, promises to spark further exploration into the relationship between light and matter in chemical reactions.
Reference: Zachary T. Brawley et al, Vibrational weak and strong coupling modify a chemical reaction via cavity-mediated radiative energy transfer, Nature Chemistry (2025). DOI: 10.1038/s41557-024-01723-6