Inside every living cell, an orchestra of proteins works in perfect—though sometimes chaotic—harmony. These proteins fold, twist, bind, and break apart in a dizzying array of configurations to keep the machinery of life humming. They are the conductors, the instruments, and the score all at once. When one of them falters, disease can follow. Cancer, Alzheimer’s, diabetes—they all share a common theme: proteins that have lost their rhythm.
For decades, drug developers have hunted for ways to interfere when this biological music goes off-key. Their strategy has often been straightforward: find the wrongdoer protein, figure out its shape, and design a drug that locks into it like a key in a door. The entire field of pharmacology has leaned on this idea—shape, structure, binding. But what happens when the protein you need to target doesn’t have a stable shape? What if it’s more like smoke than sculpture?
This is the conundrum posed by a massive portion of the human proteome—those proteins (or parts of them) that never adopt a fixed structure. Instead of standing still, they dance. These regions, known as intrinsically disordered regions or IDRs, make up more than half of all proteins in the body. Yet until now, they’ve been nearly invisible to drug development. Too slippery to hold, too wild to pin down, they’ve been written off as “undruggable.”
But science, like nature, abhors a limit. And today, thanks to artificial intelligence and the work of researchers like Nobel Prize-winning chemist David Baker and his team, those invisible proteins may finally be coming into view.
When Shape Doesn’t Matter, but Function Does
Traditionally, targeting proteins in drug development has relied on a kind of chemical matchmaking. Scientists isolate a protein linked to disease, use imaging techniques to determine its precise 3D shape, then design a molecule—often a small drug or antibody—that can bind to a pocket on that protein and modify its behavior.
But this strategy fails when the protein doesn’t have a pocket. Or worse—when the entire protein is in flux, shifting shape moment by moment. That’s exactly what happens with IDRs. These regions aren’t just loosely structured; they’re formless by design. They flop and flex, influenced by their environment and molecular companions. And yet, they are critical to life.
IDRs play key roles in how cells communicate, how genes are turned on or off, and how proteins interact with one another. They are central players in the most delicate conversations of biology—and when they malfunction, the consequences are devastating. Misfolded or disordered proteins have been implicated in a wide range of diseases, including neurodegenerative disorders like Parkinson’s and ALS, certain cancers, and autoimmune diseases.
But scientists haven’t ignored them for lack of interest. They’ve ignored them because they had no tools to address them—until now.
Turning the Problem Inside Out
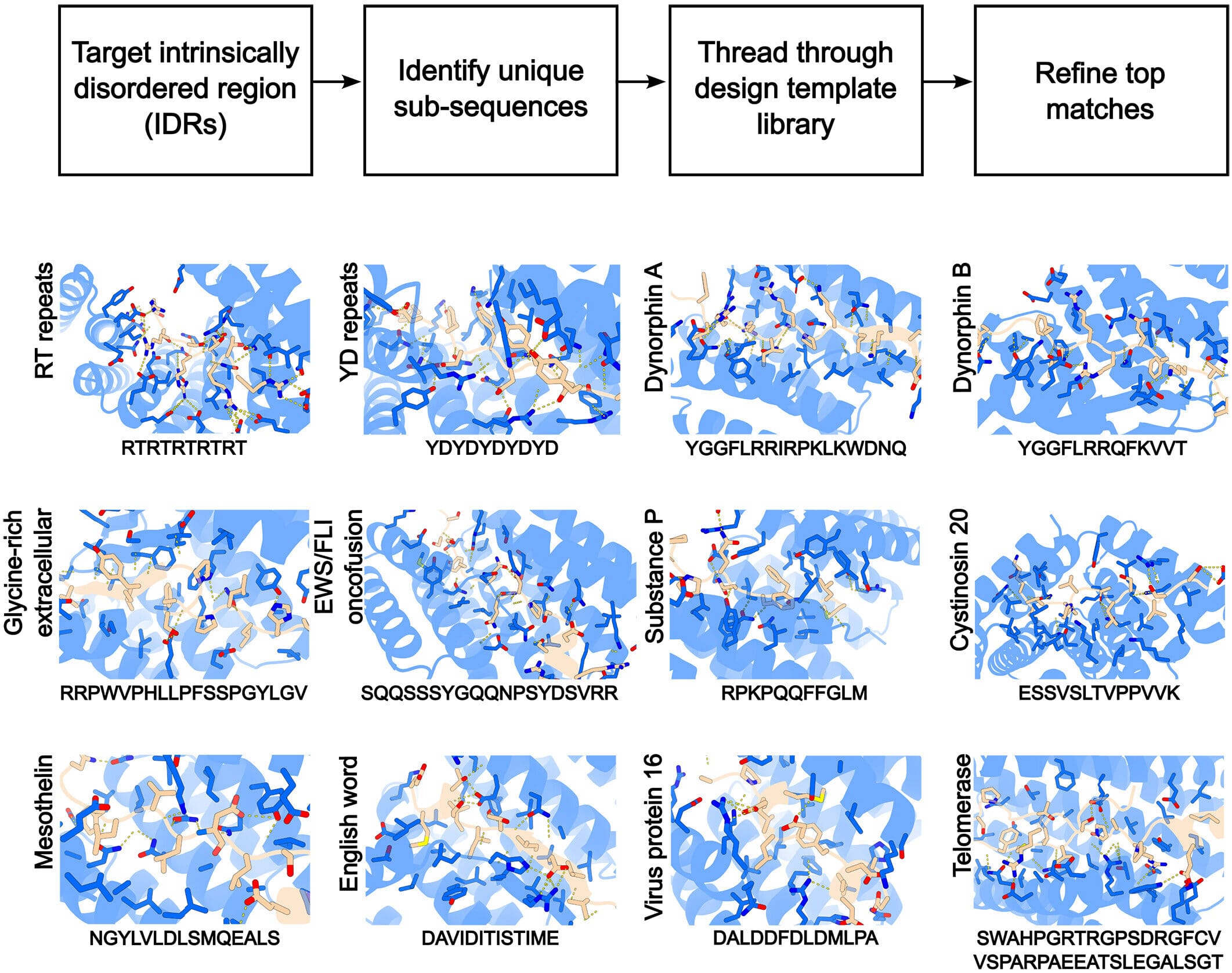
In a groundbreaking paper published in Science, Baker’s team introduced a new method that doesn’t try to fight the fluid nature of IDRs but instead embraces it. The technique, intriguingly named “Logos,” represents a complete reimagining of what it means to “target” a protein.
Rather than sculpting a molecule to squeeze into a protein’s shape, Logos flips the strategy. It creates a binding protein that acts as a welcoming pocket—one that invites the slippery, formless target to settle into it. The genius of the design is that the target protein doesn’t need to hold its shape beforehand. The binding protein induces that shape by offering a kind of chemical haven where the IDR can finally rest. It’s a little like giving water a glass—suddenly, it takes a form.
Using this approach, Baker’s team designed successful binders for 39 different disordered proteins. Some were fragments, others full proteins, but all previously considered out of reach. And in each case, the AI-driven system didn’t merely guess—it engineered molecules that could selectively and strongly bind to their chaotic targets.
As the team wrote, “Although targeting disordered proteins has been a considerable challenge for traditional methods, we show that the disorder is an advantage.” The very quality that made these proteins so difficult to work with—their shape-shifting nature—became the key to unlocking them. The binders didn’t just hold them—they helped them become bindable.
The Role of Artificial Intelligence in Molecular Design
At the heart of this revolution lies the power of artificial intelligence. The Logos method is computationally intense, relying on machine learning models trained to predict how sequences of amino acids will behave. It’s not a simple task. Proteins are built from 20 different amino acids arranged in a myriad of combinations, and their behaviors depend on countless variables—temperature, pH, neighboring molecules, even cellular mood swings.
But with enough data and enough computing power, AI can detect patterns and possibilities that human intuition never could. Logos operates like a molecular sculptor—but one whose tools are probability curves and simulation models. It can iterate through thousands of design possibilities in seconds, modeling which binding protein sequences might fold into stable shapes that perfectly cradle their disordered partners.
The result is a marriage of biology and technology so intimate it almost feels like science fiction. Proteins once too erratic to target can now be bound with designed molecules that are, in a way, tailor-made hallucinations—never found in nature, yet perfectly suited to the task.
This is more than clever design. It’s a paradigm shift.
Why This Changes Everything in Drug Discovery
The implications of this work are vast. For decades, the pharmaceutical industry has known it was leaving huge swaths of the proteome untouched. IDRs weren’t just inconvenient; they were a barrier to progress. Entire disease pathways went unaddressed. Genes known to cause illness could not be tamed, because the proteins they produced couldn’t be targeted.
Now, with Logos and the AI-powered approach behind it, that barrier is cracking.
In practical terms, this means that diseases once thought untreatable may suddenly be in reach. For example, certain forms of leukemia involve proteins with long disordered tails that interact with gene-regulating machinery. Traditional drugs can’t reach them—but designed binders might. Or consider Alzheimer’s, where sticky, misfolded proteins like tau and amyloid-beta wreak havoc. Targeting them before they clump together has always been difficult because of their fluid nature—but now, the door is open.
Even more exciting is the speed at which this technology could move. While developing a traditional drug can take over a decade and billions of dollars, computational design allows for rapid prototyping. Binding proteins could be created, simulated, and optimized within days or weeks. It’s like going from sculpting statues with a chisel to printing them with a 3D printer.
The Frontier Ahead
As promising as Logos is, it’s still the beginning. The study proved the concept, but translating it into usable medicines will take time. Challenges remain—getting these binders into the body, ensuring they work safely in living organisms, and making sure they don’t interfere with other proteins unintentionally.
But if the history of science has taught us anything, it’s that once a locked door is opened, progress floods in.
In fact, Baker’s lab isn’t working in isolation. Around the world, scientists are joining a new movement in structural biology—one that no longer assumes that shape equals function, or that instability is a problem to be avoided. The biology of the future might look less like a blueprint and more like a dance. And researchers now have the choreography to follow it.
Artificial intelligence is the catalyst. It doesn’t replace biologists or chemists—it empowers them. It helps them see the invisible, model the uncertain, and imagine what life itself might not have evolved on its own.
From Undruggable to Unstoppable
The word “undruggable” has long cast a shadow over modern medicine. It’s a label that’s been used to draw a line—between what is possible and what is not. But as Baker’s team has shown, that line was never permanent. It was a challenge waiting for the right tools.
With Logos, artificial intelligence, and the brave new world of protein design, that challenge is finally being met.
The undruggable is no longer untouchable.
It’s becoming the future of medicine.
Reference: Kejia Wu et al, Design of intrinsically disordered region binding proteins, Science (2025). DOI: 10.1126/science.adr8063