Inside every living cell, a quiet miracle unfolds every second of every day. Glucose, the sugar that powers life, is broken down, reassembled, and transformed into the energy that fuels everything from a heartbeat to a thought. We know this story well—or at least we thought we did.
But something was missing from the picture.
Until now, scientists have largely observed metabolism as if through frosted glass—able to see its overall shape, but unable to make out its inner details. While powerful techniques like bulk metabolomics have shown what molecules are present in a tissue or organism, they’ve said very little about where those molecules actually go within cells, or how they interact with the dynamic architecture of the living body.
That boundary has just been shattered.
In a groundbreaking study published in Nature Communications, a team of researchers from Vanderbilt University and the University of California, San Diego has captured the most detailed metabolic map yet—an intricate, high-resolution picture of how cells respond to glucose, not just chemically but spatially, structurally, and in real time. For the first time, scientists can now trace how glucose travels through the body down to the level of individual organelles inside single cells.
And what they’ve found reveals a universe far more complex—and far more beautiful—than anyone imagined.
Peering Through the Cellular Kaleidoscope
The research team, led by Dr. Rafael Arrojo e Drigo, assistant professor of molecular physiology and biophysics at Vanderbilt, knew that understanding metabolism in its full complexity meant embracing its full context. Cells do not exist in a vacuum. They’re nestled within tissues, which form organs, which together orchestrate life. The molecules they process move through them like musicians through a symphony—coordinated, responsive, and astonishingly precise.
But standard techniques for studying metabolism are like listening to a symphony with earmuffs on. They record averages, smoothing over variation. They don’t show which cell is playing which note or how different parts of the orchestra coordinate in real time. For years, that limitation has frustrated researchers trying to understand how metabolism goes awry in diseases like diabetes, obesity, cancer, and neurodegeneration.
So, Arrojo e Drigo’s team built something entirely new: a multi-modal, multi-scale imaging pipeline that could watch metabolism unfold in stunning detail—from entire animals down to subcellular compartments like mitochondria and lipid droplets.
By combining stable isotope tracing, advanced light and electron microscopy, mass spectrometry imaging, and artificial intelligence, they created a kind of metabolic GPS—a system that could not only tell them where glucose atoms ended up, but how their fate was shaped by the internal geography of the cell.
Rewriting the Metabolic Atlas
To chart this hidden terrain, researchers began by infusing live mice with glucose molecules labeled with stable isotopes—carbon atoms that can be tracked like tiny beacons as they move through the body. The glucose circulated through the bloodstream, was taken up by cells, and broken down or stored depending on the animal’s nutritional state.
Using an array of imaging techniques and computational models, the scientists followed these labeled glucose atoms from the liver into individual hepatocytes—liver cells known for their role in nutrient storage and detoxification. What they found was a revelation.
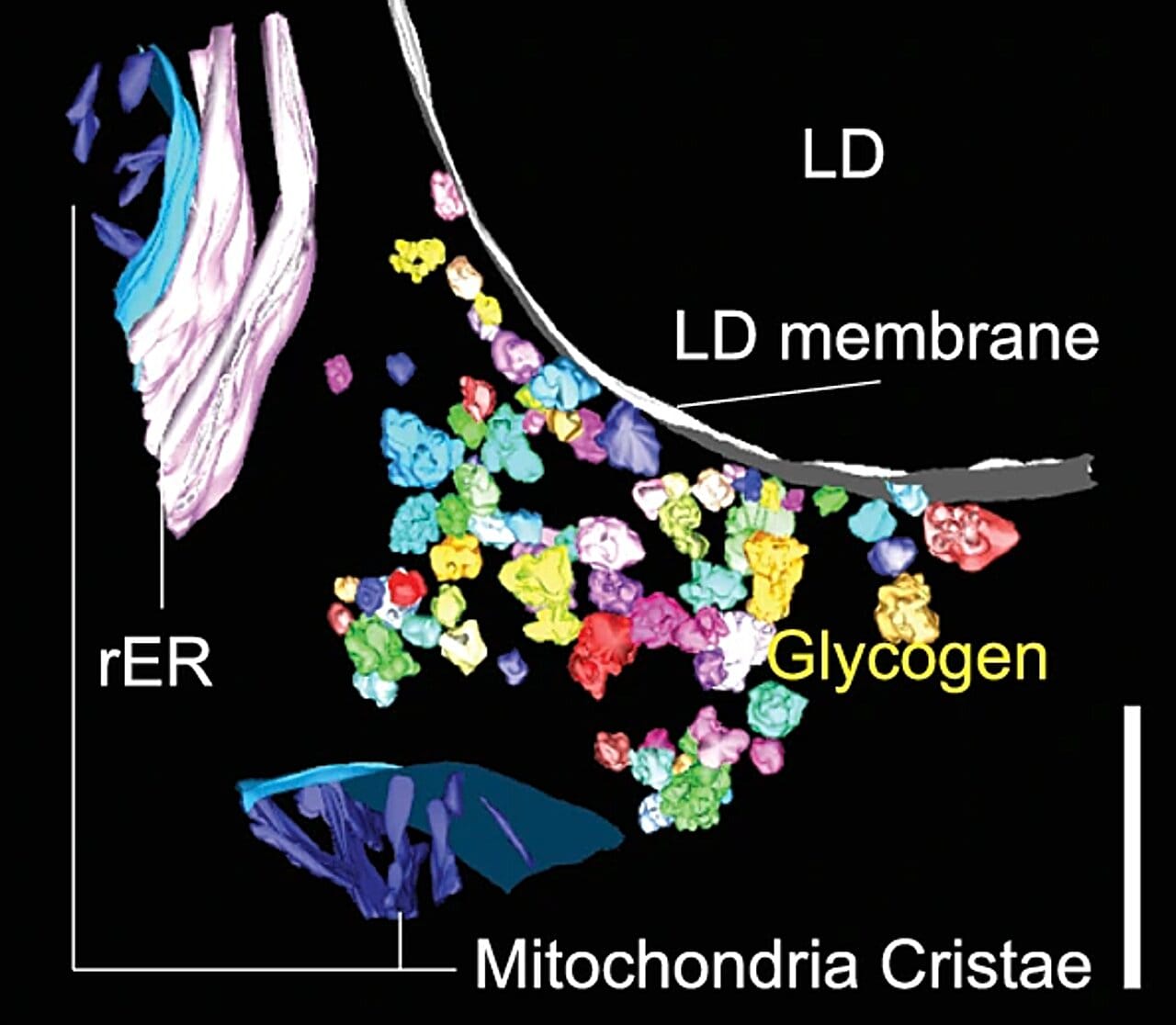
In traditional textbooks, glucose enters a cell and either gets stored as glycogen or broken down for energy. But under the microscope, the process turned out to be far more orchestrated. Glucose-derived carbons didn’t just diffuse aimlessly through the cytoplasm. They were ferried and concentrated with a surprising degree of spatial control, often in direct association with specific organelles.
Even more astonishing was the discovery of a new structural and functional partnership between lipid droplets—fat storage organelles—and sites of glycogen synthesis. These two energy storage systems, once thought to operate largely independently, were revealed to be in intimate contact, suggesting a coordinated dance between sugar and fat that had never been appreciated before.
The Mitochondria Whisper to the Endoplasmic Reticulum
One of the most striking revelations of the study was the dynamic relationship between mitochondria—the powerhouses of the cell—and the endoplasmic reticulum (ER), the sprawling membrane network involved in protein folding and lipid synthesis. These two organelles have long been known to communicate, but the new imaging showed that their interaction is not static. It shifts dramatically in response to glucose levels.
When blood glucose was high, mitochondria and ER huddled closer together, forming tight junctions that likely facilitated rapid nutrient sensing and energy production. When glucose waned, the distance increased, hinting at a metabolic “quiet mode” that may help conserve resources or shift the cell’s priorities.
These interactions weren’t random—they formed part of a larger, intricately choreographed network in which organelles continuously rearranged themselves to optimize energy use and storage. Like a factory retooling its assembly line in real time, the cell seemed to reconfigure itself depending on its nutritional state. It wasn’t just metabolizing glucose. It was adapting to it.
From Molecule to Meaning
What makes this study so powerful isn’t just the technical achievement—though the multi-scale imaging pipeline represents a significant leap forward in cell biology. It’s the conceptual breakthrough: metabolism is not merely a chemical process. It is a spatially organized system woven into the architecture of the cell itself.
By illuminating this structure-function relationship, the research offers profound new insights into how the body maintains metabolic balance—and how that balance is disrupted in disease.
In diabetes, for instance, cells lose their ability to respond properly to glucose. The current study raises the possibility that this failure may not just be about faulty signaling pathways, but about deeper disruptions in the physical layout of the cell’s metabolic machinery. If the choreography of organelles is off, even perfect glucose might not be processed correctly.
Similarly, in cancer cells, which are notorious for hijacking glucose to fuel rapid growth, understanding the spatial layout of metabolic processes could reveal new vulnerabilities—specific organelle interactions or structural dependencies that could be targeted with drugs.
Even aging, long known to alter metabolism, may involve subtle shifts in how organelles interact. Over time, the dance might slow down, the connections loosen, the harmony fade. With the tools developed in this study, scientists can now watch that symphony unravel in unprecedented detail.
A Vision for the Future
The impact of this research stretches beyond its immediate findings. By integrating AI, stable isotopes, and cutting-edge imaging, it sets the stage for a new era in biology—one where metabolism can be studied not just as a set of reactions, but as a living, breathing system embedded in the architecture of life.
Dr. Arrojo e Drigo describes the work as the foundation of a new investigative frontier—one that could unlock secrets of metabolic resilience, organ-specific energy regulation, and the molecular choreography of health and disease.
His team, along with collaborators from UCSD’s National Center for Microscopy and Imaging Research, is now working to apply these techniques to other organs, diseases, and biological states. They aim to uncover how cells in the brain, pancreas, muscle, and fat coordinate their metabolic functions—and how those relationships break down under stress, inflammation, or aging.
The Heart of the Cell, Illuminated
At its core, this study is a testament to the power of seeing. For decades, scientists have understood the chemistry of metabolism. Now, they’re beginning to understand its geometry. And in that shift—from molecules to maps, from reactions to relationships—they’re finding something unexpectedly poetic.
The cell, it turns out, is not a static container for life. It is a world in motion, an intricate cosmos of moving parts that respond to every breath we take, every bite we eat, every beat of the heart. And glucose—the humble sugar we’ve come to associate with energy—is not just fuel. It’s a traveler. A signal. A storyteller.
With every new map, we understand that story a little better.
Reference: Aliyah Habashy et al, Spatial patterns of hepatocyte glucose flux revealed by stable isotope tracing and multi-scale microscopy, Nature Communications (2025). DOI: 10.1038/s41467-025-60994-w