In a world craving change, milk is quietly undergoing a revolution. Not on pastoral farms or in glass bottles left on doorsteps, but inside gleaming laboratories where scientists are harnessing the power of microbes to do something extraordinary—produce milk proteins without a single cow in sight.
This isn’t science fiction. This is biotechnology at its most compassionate and clever. By genetically reprogramming Escherichia coli, a commonly studied bacterium, researchers have achieved something thought to be impossible only a decade ago: the microbial production of casein, the primary protein found in dairy milk. This advance could redefine everything we know about cheese, yogurt, and other milk-based products—without the environmental toll or ethical dilemmas of traditional dairy farming.
The Case for Casein: Why This Protein Matters
At the heart of milk’s magic lies casein, a family of proteins that give milk its rich texture, nutritional value, and structural versatility. Whether you’re stirring cream into coffee or slicing into a block of aged cheddar, casein is doing the heavy lifting. It’s what enables milk to carry calcium, form stable emulsions, and hold together in creamy, satisfying forms that plant-based milks often fail to replicate.
For humans, casein is more than just a functional ingredient. It’s a nutritional powerhouse—especially important in the diets of infants, athletes, and anyone seeking high-quality protein. Packed with essential amino acids and known for its slow digestion, it helps build muscle, stabilize energy levels, and support bone health through calcium delivery. Its ability to bind calcium into bioavailable forms makes it particularly valuable, both nutritionally and structurally.
But producing casein through traditional dairy means milking cows—millions of them. And the cost isn’t just in feed or land. It’s in methane emissions, water overuse, and the systemic animal suffering embedded in industrial dairy production. The $2.7 billion global casein market in 2023 came with a carbon footprint that’s increasingly hard to ignore. The call for dairy alternatives isn’t just a trend—it’s an environmental and ethical necessity.
Engineering a Better Tomorrow—Inside a Test Tube
For years, the idea of replacing dairy with plant-based alternatives has held promise but limited payoff. Almond, soy, oat, and rice milks offer cruelty-free options, but when it comes to taste, texture, and nutrition—especially in products like cheese or yogurt—they often fall short. What was missing wasn’t creativity. It was casein.
Enter the world of microbial cell factories, where microorganisms are transformed into biochemical workhorses. The food and pharmaceutical industries have long used microbes to produce vitamins, enzymes, and supplements. So why not milk proteins?
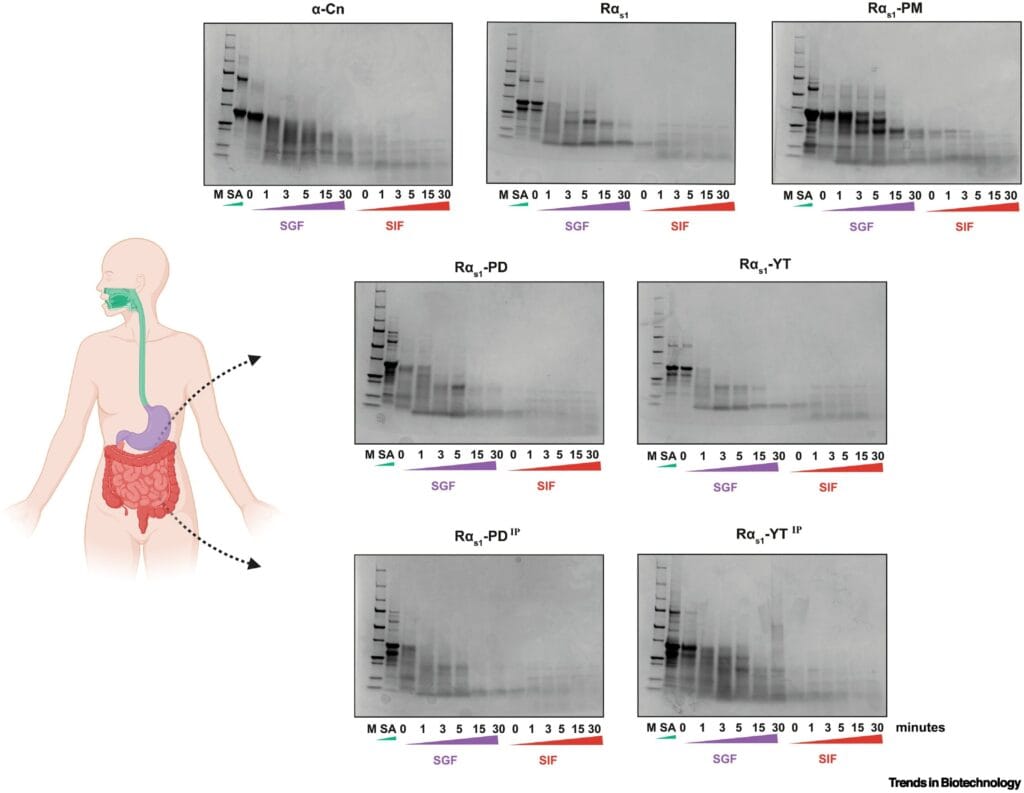
Inspired by this possibility, scientists recently pushed the boundaries of synthetic biology, publishing their findings in the journal Trends in Biotechnology. Their mission: to create nutritionally and functionally equivalent caseins using bacteria, without relying on animals at all.
But this process wasn’t as simple as inserting a gene and flipping a switch. A significant challenge stood in their way—phosphorylation.
Cracking the Code of Calcium Binding
Casein’s ability to bind calcium, form micelles, and stabilize milk comes from a precise molecular tweak: phosphorylation. This biochemical process adds a phosphate group to specific amino acids, particularly serine, altering the shape and charge of the protein. Without phosphorylation, casein doesn’t behave like it should. It won’t hold calcium, and it certainly won’t form the micellar structures that are essential to both nutrition and function.
Replicating this process outside of a cow’s body required innovation. Researchers needed to either recreate phosphorylation within bacterial systems or develop a mimic that could achieve the same effect. They chose to pursue both.
In one approach, scientists genetically engineered E. coli to co-express not just the casein gene, but also three protein kinases derived from Bacillus subtilis—enzymes responsible for catalyzing phosphorylation. These kinases enabled the bacterial host to phosphorylate the casein proteins at the right locations, mimicking the natural process that occurs in bovine mammary glands.
In parallel, they devised a phosphomimetic strategy. Instead of adding phosphate groups, they substituted specific serine residues with aspartic acid, an amino acid that mimics the negative charge of phosphorylated serine. It was a clever molecular sleight-of-hand, designed to trick biology into behaving as though real phosphorylation had taken place.
Testing the Milk of the Future
Producing casein proteins was only the first step. The real question was: do they work?
To find out, the researchers subjected the microbial caseins to a series of rigorous tests. Structural analyses confirmed that the proteins folded correctly, preserving the essential motifs seen in cow-derived caseins. Calcium-binding tests showed high affinity in both the phosphorylated and phosphomimetic versions, confirming that the essential functional behavior had been successfully replicated.
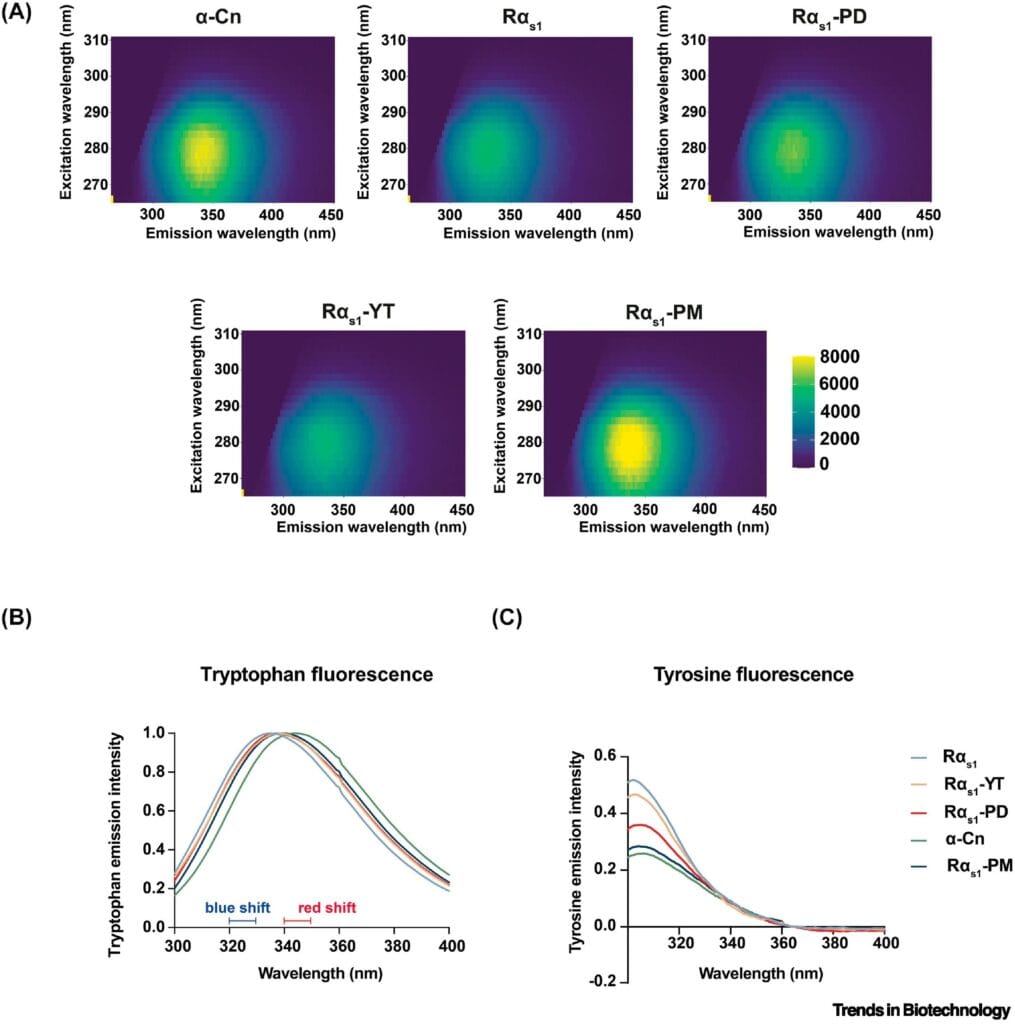
But perhaps most impressively, the proteins self-assembled into casein micelles—the microscopic protein clusters that naturally form in milk and make it such a good vehicle for nutrient delivery. These micelles ensure the even distribution of calcium and phosphorus in dairy, and their successful formation in lab-made casein is a massive win for dairy-free innovation.
To top it off, simulated digestion trials showed that the engineered caseins broke down in a similar way to natural milk proteins. This means they could be as digestible and bioavailable as their bovine counterparts, with no sacrifice to nutritional integrity.
Simplifying Complexity with Synthetic Ingenuity
While both engineered caseins showed promise, the phosphomimetic version stood out for its simplicity. Introducing kinases into bacterial systems adds metabolic burden and complexity. Phosphomimetics, on the other hand, offer a more streamlined approach for industrial-scale production. Although they may not perfectly capture every nuance of natural phosphorylation, the trade-off between ease and efficacy makes them particularly attractive for early-stage commercialization.
The research team believes that with further refinement and scaling, phosphomimetic caseins could soon serve as a foundational ingredient in a new generation of animal-free dairy products—delivering on taste, nutrition, and ethics.
But they also caution that more work remains. Precise quantification of phosphorylation, deeper understanding of casein micelle formation, and long-term safety and regulatory assessments are still ahead. Yet the groundwork has been laid. And it’s solid.
Rethinking Dairy—One Microbe at a Time
The implications of this breakthrough stretch far beyond a better vegan cheese. They signal a new era of food production—where sustainability, functionality, and ethics can finally align. Imagine dairy products that contain no allergens, are tailored for optimal nutrition, and have a shelf life engineered for global distribution—all made without harming a single animal or exhausting our planet’s resources.
This vision isn’t as far off as it once seemed. With microbial biotechnology accelerating, and with increasing public demand for cruelty-free, climate-friendly food systems, products made from recombinant caseins may soon become not just viable, but mainstream.
In a future where cows are no longer central to the dairy equation, bacteria may be the heroes no one saw coming. They’ll never graze a pasture or moo at the morning sun. But thanks to science, they just might nourish the world.
Conclusion: A Glass of Kindness, Engineered by Science
Dairy has always carried a sense of comfort—warm milk before bed, cheese on a favorite dish, yogurt spooned on a summer morning. But comfort need not come at a cost.
What this breakthrough shows is that tradition and innovation are not enemies. They can coexist. By decoding and replicating nature at the molecular level, scientists are not rejecting the essence of milk—they’re liberating it from the limitations of the past.
With microbes in the lab and compassion in mind, we are now beginning to reimagine dairy for a world that desperately needs both nourishment and change. One day soon, you may pour a glass of milk or spread butter on toast, knowing it was made not by an animal, but by human ingenuity—and by bacteria doing their quiet, extraordinary work.
Reference: Suvasini Balasubramanian et al, Production of phosphorylated and functional αs1-casein in Escherichia coli, Trends in Biotechnology (2025). DOI: 10.1016/j.tibtech.2025.05.015