A research team from the Department of Chemistry at the Ulsan National Institute of Science and Technology (UNIST) has made a groundbreaking discovery regarding the harmful effects of blue light on cellular proteins. The team’s findings, which were published in the December 2024 issue of Nature Communications, reveal a new biochemical pathway through which blue light causes oxidative damage to cellular proteins, potentially influencing health outcomes in both skin and eye tissues. This research paves the way for a deeper understanding of the risks associated with exposure to blue light and its far-reaching effects on human health.
The Widespread Sources and Characteristics of Blue Light
Blue light, a form of high-energy visible light, is abundant in both natural and artificial environments. Sunlight is the most significant natural source, but blue light is also emitted by LED-based display devices such as smartphones, tablets, computers, and TVs. Additionally, modern indoor lighting has incorporated more energy-efficient light sources that emit substantial amounts of blue light. Although essential for regulating circadian rhythms and contributing to alertness during the day, blue light also poses unintended consequences for human health, particularly when exposure becomes chronic or unregulated.
What makes blue light particularly concerning is its high energy. Unlike other forms of visible light, blue light is able to penetrate deeply through the human eye. When we look at screens for long periods of time or when we are exposed to artificial lighting at night, this energy reaches the retina, the light-sensitive tissue at the back of the eye, potentially contributing to long-term damage. More troubling is that blue light is not effectively filtered by conventional sunscreen, leaving both the skin and eyes exposed to potential harm.
Understanding the Mechanism of Damage
The effects of blue light on human health are not just speculative; studies have shown that it can induce oxidative stress, a condition in which reactive oxygen species (ROS) accumulate and damage cells. Blue light causes the oxygen dissolved in body tissues, including those in the skin and eyes, to interact with light photons, creating these highly reactive oxygen molecules. These molecules are capable of attacking and oxidizing proteins in a process known as photooxidation.
Cellular proteins are essential to the proper function of every cell in the body, playing vital roles in structure, function, and regulation. Under normal circumstances, the body’s natural antioxidant systems—complex biochemical networks designed to neutralize ROS—are sufficient to prevent or minimize protein damage. However, in the presence of continuous or intense oxidative stress, as caused by blue light exposure, these antioxidant defenses may not be enough to protect the proteins, resulting in cellular dysfunction or even cell death.
What is most striking about the recent findings of the UNIST research team is that blue light’s damaging effect on proteins occurs in a novel manner, affecting areas that were previously not considered vulnerable.
The Novel Oxygen-Defined Photooxidation Pathway
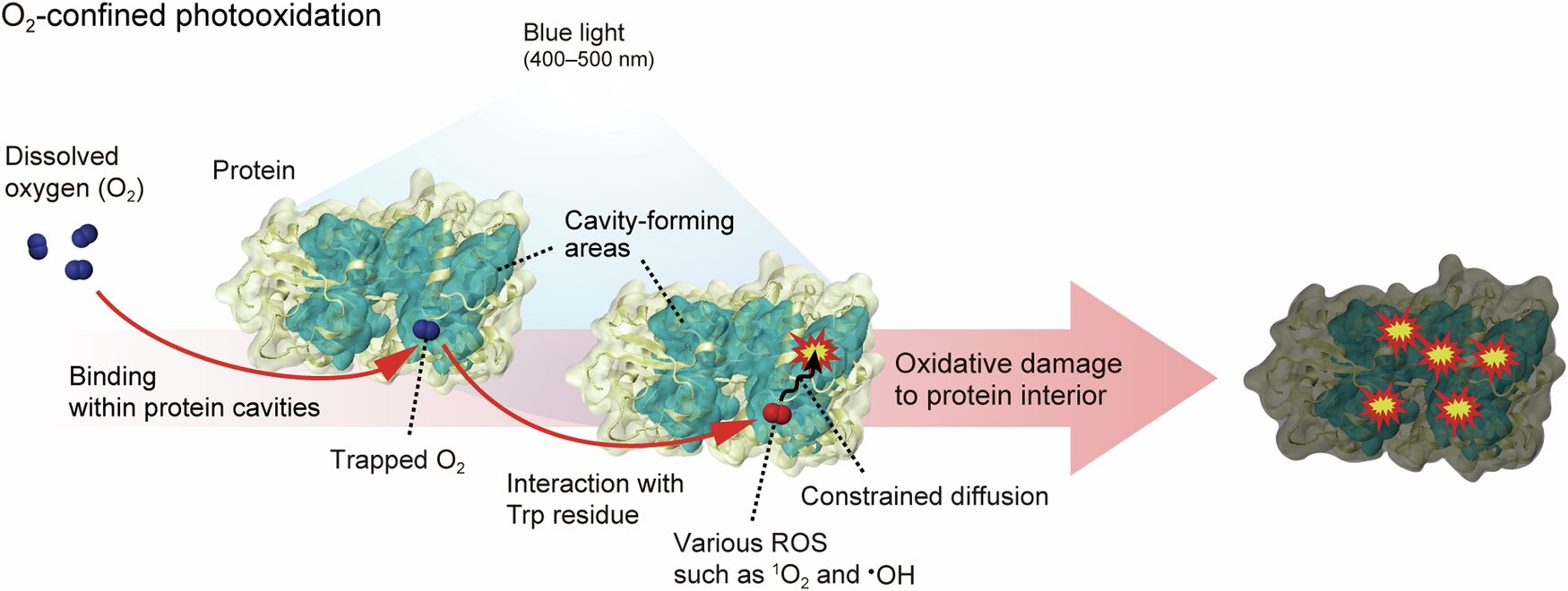
Up until this discovery, scientists understood that blue light caused damage to proteins by generating ROS that acted on exposed protein surfaces or interacted with molecules near the cell’s outer layer. However, the UNIST researchers have revealed a deeper, more insidious mechanism: blue light induces damage within the inner cavities of proteins, areas not easily accessible to traditional antioxidant defenses.
The team, led by Professors Duyoung Min, Tae-Hyuk Kwon, and Seung Kyu Min, used a variety of advanced techniques to uncover this novel protein damage pathway, which they named the “oxygen-defined photooxidation pathway.” Their findings show that in certain regions of proteins, which have an intricate folding structure, there exist cavities where oxygen can be trapped. These pockets of trapped oxygen are key to the mechanism. Upon exposure to blue light, the trapped oxygen interacts with specific amino acids within the protein, absorbing the light’s energy. The absorbed energy is then converted into ROS, which diffuse through the cavity and initiate the oxidation process, causing damage to the protein structure.
This new pathway is significant because it extends beyond the conventional understanding of cellular oxidative damage. While previous models focused on ROS generation from the external surface of proteins, this discovery suggests that even the inner structures of proteins—shielded from antioxidant systems—are vulnerable to blue light-induced damage. The team hypothesizes that this mechanism may contribute to cellular aging and the development of diseases related to skin and eye tissues, such as age-related macular degeneration (AMD) or skin aging and pigmentation disorders, following prolonged blue light exposure.
Detailed Research Approach
The research team employed a multifaceted approach to identify and confirm this pathway. First, they relied on computational methods and structural analyses to understand how the unique shape and folding of proteins might allow certain areas to trap oxygen. Using advanced bioinformatics, statistical analysis, and rigorous experimental methodologies, including fluorescence spectroscopy and molecular dynamics simulations, they were able to pinpoint how light energy interacts with specific regions within the protein cavities.
The findings were strengthened by experimental validation, where the team directly observed how blue light exposure could trigger ROS formation inside protein cavities, further implicating this as a previously unseen method by which proteins undergo oxidative damage. Their work combined both theoretical predictions with tangible experimental observations, resulting in a comprehensive understanding of the process.
This discovery may fundamentally change how scientists think about oxidative damage and protein health in a biological context. Given the pervasive presence of blue light in modern environments, understanding how and why proteins in various tissues, such as the skin and eyes, suffer damage from exposure to light could lead to innovations in treatment or prevention strategies.
Implications for Skin and Eye Health
One of the most immediate concerns stemming from this research is its potential impact on skin and eye health. Blue light’s contribution to eye conditions like AMD could be more significant than previously believed. Recent studies have already implicated blue light in retinal damage, but now, this new mechanism gives scientists an additional framework to better understand why blue light is so harmful at the cellular level. For skin health, oxidative damage from blue light can accelerate skin aging, promote pigmentation disorders, and lead to more severe long-term effects, especially since individuals are often unaware of how much blue light they are absorbing from daily activities.
This discovery could catalyze a range of new preventive measures in health and wellness, with potential for novel protective products that go beyond just sunscreen. Currently, sunscreens are designed to block harmful ultraviolet (UV) radiation, which is a known cause of oxidative damage and DNA mutations. However, because sunscreen does not effectively block blue light, additional strategies or formulations that target blue light could become essential in managing its harmful effects on the skin.
For eye care, further exploration into treatments that protect retinal cells from blue light-induced damage will be crucial. Innovations could include the development of specialized filters or antioxidant treatments that specifically address this newly identified form of oxidative stress.
Future Directions
While this research opens new avenues for protecting against blue light-related damage, it also prompts many new questions. For instance, could this “oxygen-defined photooxidation pathway” be relevant in other tissues or organs that experience light exposure? What are the long-term consequences of chronic low-level blue light exposure? And how might this mechanism play a role in conditions beyond skin aging and eye disease, such as neurodegenerative disorders or other forms of cellular aging?
Professor Duyoung Min, one of the principal investigators in the study, highlighted the implications of these findings. “We have discovered a new protein damage pathway that is fundamentally different from the conventional mechanisms of protein damage, and we have demonstrated that it can universally affect proteins throughout the cell. This newly identified protein damage pathway may represent a hidden principle contributing to aging or disease processes in skin and eye tissues due to blue light exposure.”
As scientists delve deeper into these questions, the understanding of blue light’s biological effects will continue to evolve. Armed with this knowledge, there will be exciting opportunities to explore how we can better shield ourselves from its adverse impacts, ultimately leading to better health outcomes in a world increasingly dominated by artificial light.
Reference: Seoyoon Kim et al, Hidden route of protein damage through oxygen-confined photooxidation, Nature Communications (2024). DOI: 10.1038/s41467-024-55168-z