Monoclonal antibodies (mAbs) have become cornerstone tools in modern medicine, offering groundbreaking treatments for a wide range of diseases, from cancer and Alzheimer’s disease to autoimmune disorders and infectious conditions. These laboratory-engineered proteins are designed to mimic the immune system’s ability to fight harmful pathogens. One specific monoclonal antibody, NISTmAb, has recently garnered significant attention in biopharmaceutical research due to its unique properties, particularly its ability to maintain its structural integrity even when its attached sugar molecules—known as glycans—are altered. A groundbreaking study led by Associate Professor Christian Bleiholder from the Florida State University (FSU) Department of Chemistry and Biochemistry has shed new light on this phenomenon, offering valuable insights for biopharmaceutical developers working to refine monoclonal antibody-based therapies.
The study, published in the journal Chemical Communications, marks a pivotal step in understanding the intricate dynamics of monoclonal antibodies like NISTmAb. Understanding the relationship between antibody structure and behavior is crucial in biotherapeutics since any changes in an antibody’s structure could impact its ability to target diseases effectively. This research is particularly important for the development of next-generation therapies, which require precise control over molecular structures.
NISTmAb and Its Significance in Biopharmaceuticals
Monoclonal antibodies are among the largest and most diverse groups of biologic drugs, which are derived from living organisms and engineered to treat a variety of conditions. These biologic drugs are made by creating identical copies (clones) of a single type of immune cell called a B cell that produces the antibody. Once produced, monoclonal antibodies are used to treat diseases by targeting specific proteins in the body, such as those involved in cancer cell growth or inflammation in autoimmune diseases.
A key challenge in monoclonal antibody-based therapies is ensuring the protein maintains its correct structure. The structure of an antibody is vital to its ability to bind to target molecules or pathogens. Glycosylation, the process by which sugar molecules attach to proteins, plays a critical role in protein structure and function. In the past, there has been significant debate over how changes in glycosylation affect monoclonal antibodies. This new research highlights that modifying the glycosylation of NISTmAb does not appear to affect its overall structure or function, a discovery with profound implications for biotherapeutic development.
This finding is particularly important because it could make the manufacturing process of monoclonal antibodies more efficient. If glycosylation does not significantly impact the structure or efficacy of the antibody, developers can focus on optimizing other aspects of the antibody’s design, speeding up the drug development process and potentially lowering costs.
Tandem-TIMS: A Revolutionary Technique in Protein Analysis
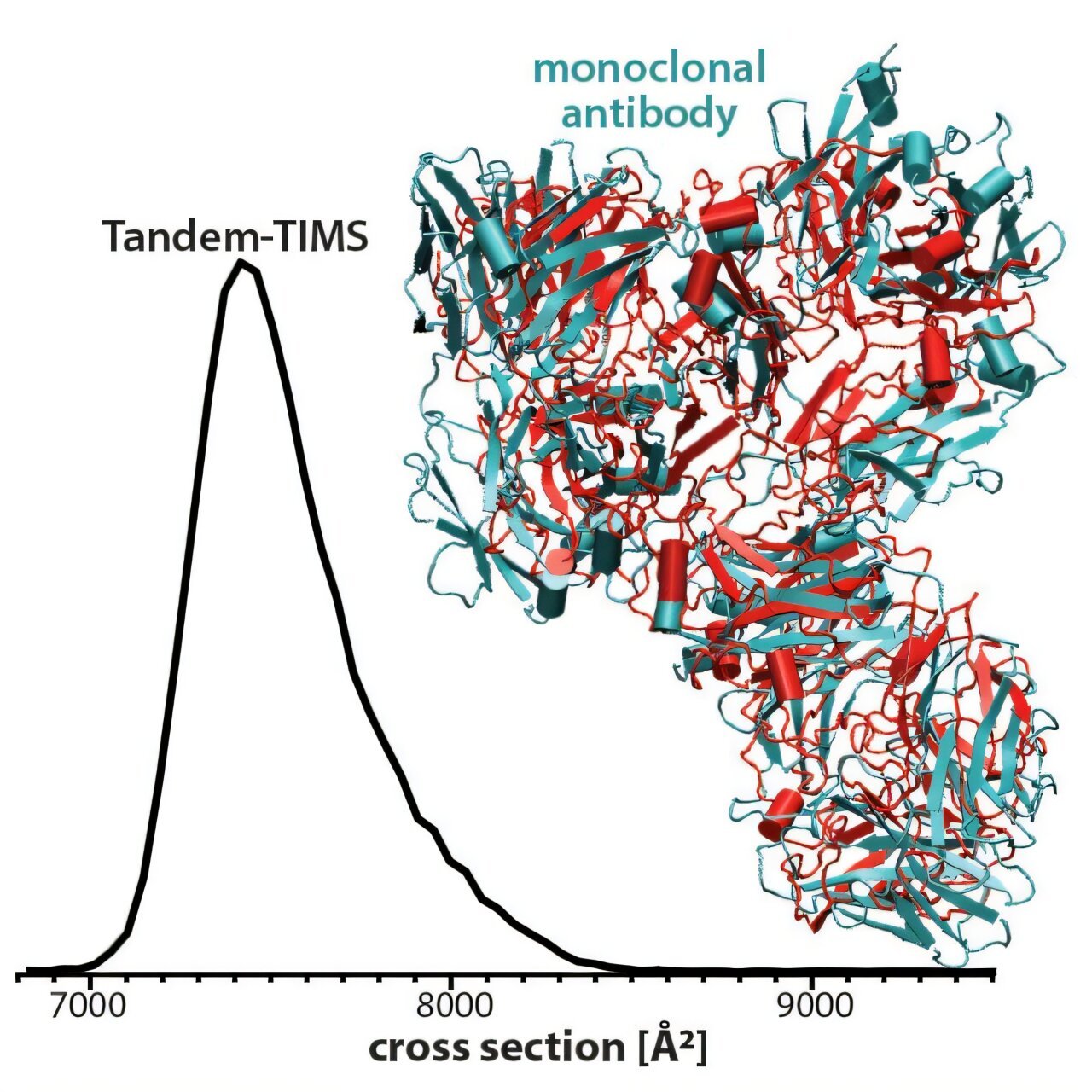
To understand how glycosylation affects NISTmAb, Bleiholder and his collaborators employed a novel analytical technique known as Tandem-TIMS (Tandem-Trapped Ion Mobility Spectrometry). This technique, developed in Bleiholder’s lab, allows scientists to examine the structure of proteins with an unprecedented level of detail while preserving their integrity. Traditional methods of protein analysis often involve harsh conditions that can lead to structural changes, making it difficult to study the protein’s natural form. Tandem-TIMS overcomes this limitation by allowing researchers to study proteins in conditions that closely mimic their biological environment, preserving their 3D structure.
The development of Tandem-TIMS has been a decade-long endeavor. Bleiholder first encountered ion mobility mass spectrometry during his postdoctoral research at the University of California, Santa Barbara, where he used it to study molecular changes related to Alzheimer’s disease. By 2013, after establishing his lab at FSU, Bleiholder began refining the technique to create a tool capable of providing deeper insights into complex proteins, such as monoclonal antibodies. The result was Tandem-TIMS, a breakthrough technology that has already begun to transform protein research.
By isolating different subpopulations of the NIST monoclonal antibody, Bleiholder’s team was able to determine that the glycosylation process—the attachment of sugar molecules—does not significantly alter the antibody’s structure or its ability to bind to its target. This finding was critical in demonstrating that sugar modifications do not introduce variability in antibody behavior, a major concern for the biopharmaceutical industry.
Implications for Biotherapeutics and Drug Development
The discovery that glycosylation does not alter the fundamental structure of NISTmAb has significant ramifications for the biopharmaceutical industry. Biotherapeutic drugs are subject to strict regulatory scrutiny, and their development often requires extensive testing and validation. The ability to modify antibodies without disrupting their structure could help researchers streamline the design process, reducing development time and increasing the consistency of the therapeutic antibodies.
Furthermore, the use of Tandem-TIMS to analyze monoclonal antibodies could aid in the development of more effective and safer treatments. By enabling scientists to better understand how antibodies behave under different conditions, this technology could pave the way for the creation of antibodies that are more stable, more selective in targeting diseases, and more effective in treating patients.
In addition, this research could have broader applications for the design of other biologic drugs. For example, understanding the structural stability of antibodies and other proteins will help accelerate the development of personalized medicine, where treatments are tailored to individual patients based on their genetic and molecular profiles. As biopharmaceutical companies continue to explore new therapies for a range of diseases, from cancer to infectious diseases, tools like Tandem-TIMS will be essential in optimizing drug efficacy and safety.
Collaboration with Industry Leaders
The impact of Bleiholder’s research is already being felt beyond the academic world. The work has sparked collaborations with industry leaders, including Johnson & Johnson, as they explore how Tandem-TIMS can be used to enhance the development of new biotherapeutic drugs. By applying this technology to real-world drug development, these collaborations could help accelerate the timeline for bringing monoclonal antibody-based treatments to market, ultimately benefiting patients who rely on these therapies for conditions like cancer, autoimmune diseases, and more.
In addition to his work on monoclonal antibodies, Bleiholder’s lab is expanding the applications of Tandem-TIMS to include the analysis of other crucial proteins, including the SARS-CoV-2 spike protein and the HIV capsid assembly. By understanding the structural properties of these proteins, researchers can develop more targeted and effective treatments for infectious diseases. The ability to study these proteins in greater detail could also lead to advances in vaccine design and antiviral therapies, which have become increasingly important in the wake of the COVID-19 pandemic.
Moreover, Bleiholder’s lab is working on developing new methods for imaging proteins in tissue samples, which could lead to major advances in personalized medicine. This approach could allow clinicians to design treatments that are tailored to the individual patient’s tissue and protein profiles, making therapies more effective and reducing the risk of adverse side effects.
Conclusion
The research conducted by Christian Bleiholder and his team at Florida State University represents a major leap forward in the field of protein dynamics and the development of monoclonal antibody therapies. By demonstrating that glycosylation does not affect the structure of NISTmAb, the study offers valuable insights that will help streamline the design and development of monoclonal antibody-based drugs. The application of the Tandem-TIMS technique in protein analysis has already proven to be a game-changer in biopharmaceutical research, and it holds great promise for accelerating drug development, improving treatment outcomes, and advancing personalized medicine. With continued collaboration between academic researchers, industry leaders, and medical professionals, these innovations could soon lead to more effective therapies for patients around the world, revolutionizing the way we treat a wide range of diseases.
Reference: Fanny C. Liu et al, Differential glycosylation does not modulate the conformational heterogeneity of a humanised IgGk NIST monoclonal antibody, Chemical Communications (2024). DOI: 10.1039/D4CC02125H