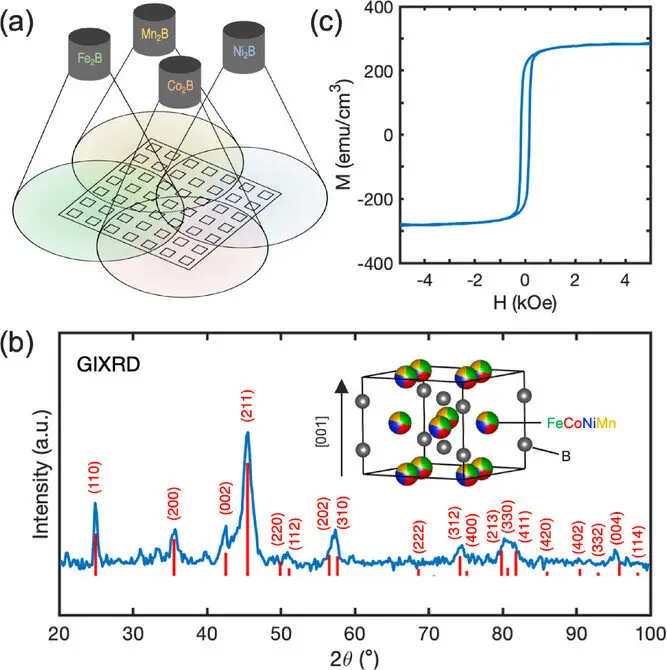
In the early 20th century, physicists began to probe deeper into the heart of matter, searching for the ultimate building blocks of the universe. Until that time, atoms were thought to be indivisible—after all, the word “atom” comes from the Greek word atomos, meaning “uncuttable.” But discoveries in the subatomic realm shattered this notion. Scientists came to understand that atoms themselves were composed of even smaller constituents: protons, neutrons, and electrons. Then, in a pivotal moment that would reshape history and redefine human power, it was discovered that atoms could, in fact, be split. This process, called nuclear fission, would unlock a previously inconceivable source of energy and usher in a new age—one of both tremendous promise and grave peril.
Nuclear fission is the process by which the nucleus of an atom splits into two or more smaller nuclei, accompanied by the release of a large amount of energy. This seemingly simple event lies at the heart of nuclear reactors and atomic bombs. It is the principle that powers submarines silently beneath oceans and produces electricity for cities. Yet it is also the mechanism behind weapons of unimaginable destructive force. To understand nuclear fission is to explore the delicate balance between knowledge and power, between innovation and responsibility.
The Structure of the Atom: A Universe Within
To grasp how nuclear fission works, one must first understand what makes up an atom. At the center of every atom lies a dense nucleus composed of protons and neutrons. Surrounding this core, electrons orbit in a cloud, held in place by the electromagnetic force. But it is the nucleus that concerns us when discussing nuclear fission.
Protons carry a positive charge, while neutrons are neutral. The force that binds protons and neutrons together in the nucleus is called the strong nuclear force. This force is incredibly powerful but acts only over very short distances. Within stable nuclei, the strong nuclear force is enough to overcome the natural repulsion between positively charged protons. But as the number of protons increases—as in the heavier elements like uranium and plutonium—the nucleus becomes more unstable, and fission becomes a possibility.
The Discovery of Fission: A Scientific Earthquake
The concept of nuclear fission emerged in the late 1930s, during a period of intense research and experimentation in nuclear physics. In 1938, German chemists Otto Hahn and Fritz Strassmann bombarded uranium atoms with neutrons. To their astonishment, they found that one of the resulting products was barium, an element much lighter than uranium. How could uranium transform into barium?
The theoretical explanation was provided by physicist Lise Meitner and her nephew Otto Frisch. They proposed that the uranium nucleus had split into two smaller nuclei, releasing energy in the process. They called this phenomenon “fission,” drawing an analogy to biological cell division. The idea was revolutionary. Einstein’s famous equation, E=mc², indicated that mass could be converted into energy. In fission, a tiny amount of mass was indeed lost—but the energy released was enormous.
The Physics of Fission: Breaking It Down
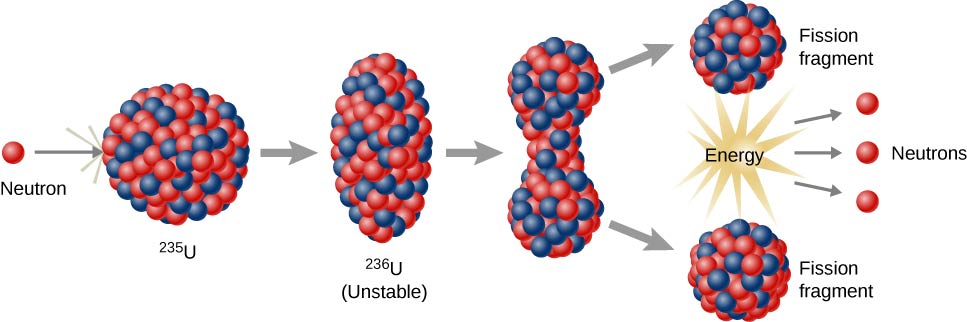
At the core of fission lies the behavior of neutrons—uncharged particles that can slip into atomic nuclei without being repelled by electric forces. When a neutron strikes a heavy nucleus like uranium-235 or plutonium-239, it can destabilize the nucleus, causing it to deform and eventually split into two smaller nuclei. These fragments are called fission products and are themselves radioactive, often decaying further over time.
The splitting of the nucleus releases several new neutrons, typically two or three. These neutrons can go on to strike other nuclei, triggering additional fission reactions. This sets the stage for a chain reaction, a self-sustaining series of fissions that can produce a tremendous amount of energy.
The key to controlling this chain reaction lies in managing the neutrons. If each fission leads to exactly one more fission, the reaction is steady. If more than one neutron causes further fission, the reaction grows exponentially. If fewer, it dwindles. In nuclear reactors, control rods made of materials like cadmium or boron absorb excess neutrons, keeping the reaction at a steady rate. In nuclear weapons, the goal is an uncontrolled chain reaction—an explosive release of energy.
Energy Release: A Nuclear Bonfire
Why does fission release so much energy? The answer lies in binding energy—the energy that holds a nucleus together. When a heavy nucleus like uranium-235 splits, the total binding energy of the resulting fragments is greater than that of the original nucleus. The difference in energy is released in the form of kinetic energy of the fragments, gamma rays, and the energy of the emitted neutrons.
The amount of energy from a single fission event is minuscule—around 200 million electron volts (MeV). But because of the vast number of atoms in even a small amount of material, the total energy is staggering. A single gram of uranium-235, if completely fissioned, can release as much energy as burning several tons of coal.
Controlled Fission: Powering the World
Harnessing nuclear fission for peaceful purposes led to the development of nuclear reactors. These complex machines are designed to control the fission chain reaction, using the heat generated to produce steam, which then drives turbines to generate electricity.
The first nuclear reactor, the Chicago Pile-1, was constructed under the stands of a football stadium at the University of Chicago in 1942, under the leadership of physicist Enrico Fermi. It was a crude assembly of uranium, graphite, and control rods, but it proved that a sustained, controlled fission reaction was possible.
Modern reactors are vastly more sophisticated, incorporating layers of safety mechanisms, containment structures, and cooling systems. They use fuel rods containing enriched uranium or plutonium, moderators like water or graphite to slow neutrons, and control rods to regulate the chain reaction.
The advantages of nuclear power are significant. It produces no greenhouse gas emissions during operation, offers high energy density, and can provide a steady, reliable supply of electricity. However, it also presents challenges: radioactive waste, risk of accidents, high capital costs, and concerns over proliferation.
Uncontrolled Fission: The Atomic Bomb
While nuclear power plants aim to control fission, atomic bombs harness its explosive potential. The first nuclear weapons were developed during World War II under the secretive Manhattan Project. Scientists like Oppenheimer, Fermi, and Szilard raced against Nazi Germany to unlock the destructive power of the atom.
The bombs dropped on Hiroshima and Nagasaki in 1945—”Little Boy” and “Fat Man”—were fission weapons. Little Boy used uranium-235, while Fat Man used plutonium-239. In both cases, a rapid, uncontrolled chain reaction was initiated, releasing energy equivalent to tens of thousands of tons of TNT in a fraction of a second. The cities were devastated, and tens of thousands of lives were lost instantly, with many more suffering from radiation effects in the years to come.
These events marked the dawn of the atomic age, a period characterized by both awe and fear. Nuclear weapons changed the geopolitical landscape, ushering in an era of deterrence, arms races, and existential dread.
The Aftermath: Radiation and Waste
Fission leaves behind a legacy—not just in energy, but in radioactive waste. The fission products generated are often highly radioactive and remain hazardous for thousands of years. Managing this waste safely is one of the biggest challenges facing the nuclear industry.
Spent nuclear fuel is initially stored in pools of water to cool and shield against radiation. Eventually, it may be transferred to dry casks or reprocessed to extract usable materials. Long-term solutions include geological repositories, such as the proposed Yucca Mountain site in the United States, though these projects are fraught with political and environmental controversy.
In addition to waste, fission poses risks of radiation exposure. Accidents like Chernobyl (1986) and Fukushima (2011) have underscored the potential consequences of reactor failures. While modern reactors are far safer and incorporate multiple layers of protection, the specter of disaster lingers.
Fission vs. Fusion: The Nuclear Divide
It’s important to distinguish between nuclear fission and nuclear fusion. Fission involves splitting heavy atoms; fusion involves combining light ones, like hydrogen, to form helium. Fusion is the process that powers the sun and promises even greater energy potential with fewer risks.
However, achieving controlled fusion on Earth has proven elusive. The extreme temperatures and pressures required are difficult to sustain. Projects like ITER and the National Ignition Facility are making progress, but commercial fusion power remains a dream for the future.
For now, fission remains the only practical way to tap nuclear energy for electricity, though research into safer, more efficient fission technologies continues.
Modern Innovations: The Next Generation of Fission
The future of nuclear fission may lie in advanced reactors—smaller, safer, and more flexible designs known as Small Modular Reactors (SMRs) and Generation IV reactors. These systems aim to reduce waste, enhance safety, and use fuel more efficiently.
Some designs, like molten salt reactors and fast breeder reactors, promise to consume nuclear waste or operate at higher efficiencies. Others are being designed to pair with renewable energy sources to provide stable grid support.
New fuel types, better materials, and digital control systems are also being explored to rejuvenate nuclear energy’s image and role in a carbon-constrained world. While the path is not without obstacles, many experts believe that nuclear fission, reimagined, can play a critical role in the global energy transition.
Philosophy, Ethics, and the Atom
Nuclear fission isn’t just a scientific or engineering feat—it is a profound ethical and philosophical frontier. The ability to split the atom has given humanity godlike power, capable of reshaping civilizations or destroying them.
Questions abound: Who controls this power? How should it be used? Can we trust ourselves to wield it responsibly? These issues are not just technical—they are deeply human.
Einstein himself, whose work laid the groundwork for nuclear energy, later expressed deep regret over the weaponization of atomic power. He spent his final years advocating for peace and disarmament, warning that the splitting of the atom had changed everything—except our ways of thinking.
Conclusion: The Legacy of Fission
Nuclear fission is a marvel of modern science, a gateway into the heart of matter and a testament to human ingenuity. It has brought light to cities and shadows to history. It is a reminder of our capacity for both creation and destruction.
To understand fission is to see the duality of knowledge—the brilliance and the burden. As we look to a future shaped by climate challenges, technological leaps, and geopolitical complexities, the lessons of nuclear fission remain vitally relevant. We must continue to explore, question, and innovate—but always with wisdom and humility.
In splitting the atom, we found not just energy, but a mirror reflecting our deepest hopes and fears. The true power of nuclear fission lies not just in the energy it releases, but in what it reveals about us.