Every time a new life begins, it inherits half of its genetic material from each parent. This dance of DNA follows the rules laid out by Gregor Mendel more than a century ago: genes come in pairs, and each parent contributes just one copy—or allele—to their offspring. But deep within the cells responsible for reproduction, not all alleles play fair.
Some alleles cheat. These so-called “meiotic drivers” bend the rules to increase their own chances of being passed on, often harming the organism along the way. In a fascinating new discovery, researchers from the Whitehead Institute and MIT have identified a selfish gene in fruit flies that cheats—but then reins itself in to avoid destroying its host species. Their work sheds new light on the evolutionary arms race waged in the very cells responsible for life.
A Gene That Plays Dirty: Introducing Stellate
The protagonist of this molecular drama is a gene called stellate (Ste), found on the X chromosome of Drosophila melanogaster, the common fruit fly. The lab of Whitehead Institute Member Yukiko Yamashita, in collaboration with graduate student Xuefeng Meng, has found that Ste acts as a meiotic driver—an allele that hijacks the rules of genetic transmission to favor its own passage to the next generation.
Unlike typical genes that passively follow the rules, Ste engages in an act of sabotage. It damages sperm that don’t carry it, specifically targeting Y-bearing sperm. By doing so, it increases the proportion of X-bearing sperm that survive and fertilize eggs, skewing the offspring toward females. In evolutionary terms, Ste is a ruthless winner.
But nature, as it turns out, has a built-in sense of balance. This selfish gene also seems to know when to pull back.
Meiotic Drivers and the Perils of Success
In normal male meiosis—the process by which sperm are formed—germline cells undergo two rounds of division to produce sperm cells, each carrying either an X or Y chromosome. Ideally, this results in a 50/50 sex ratio. But when meiotic drivers intervene, they can distort this balance.
Ste isn’t the only gene with such ambitions. Many meiotic drivers have been identified in various species, and most follow a similar path: destroy gametes that don’t carry them, usually targeting those with the opposite sex chromosome. In the case of Ste, the gene targets Y-bearing sperm, threatening to flood the next generation with females.
At first glance, this might seem like an evolutionary advantage. More females mean more potential offspring. But there’s a catch: too few males, and a population could collapse. This is the paradox of “fatal success”—when a selfish gene wins so completely that it dooms the species that carries it.
Silencing the Saboteur: A Role for piRNAs
To counterbalance Ste‘s destructive behavior, evolution has equipped the Y chromosome with a secret weapon: the suppressor of stellate (Su(Ste)), a gene that produces tiny RNA molecules known as piRNAs. These piRNAs team up with specialized proteins to silence Ste‘s RNA before it can cause damage. It’s a delicate tug-of-war: if Ste gains the upper hand, the male’s sperm become unviable. If Su(Ste) prevails, the balance is restored.
But this defense is not perfect. Since Ste and Su(Ste) reside on different chromosomes—X and Y, respectively—they can be separated during reproduction. A male offspring might inherit Ste without its corresponding suppressor, allowing the selfish gene to act unchecked.
Yet, even in the absence of its suppressor, Ste doesn’t completely take over. The expected flood of female offspring never arrives. Why not?
The Mystery of Self-Restraint
To unravel this puzzle, the Yamashita lab dug deeper into sperm development. They discovered that Ste expression starts subtly—early germ cell development proceeds normally even in the presence of the selfish gene. Trouble starts later.
As sperm cells mature, they must package their DNA using special proteins called protamines, which tightly wrap DNA into a stable, compact form. Without protamines, the sperm’s DNA becomes unstable and unusable.
The researchers found that in males with active Ste, some spermatids—immature sperm—failed to incorporate protamines properly. Intriguingly, these defective sperm were disproportionately Y-bearing. This indicated that Ste‘s sabotage was real—but only partial.
Asymmetry in a Symmetrical World
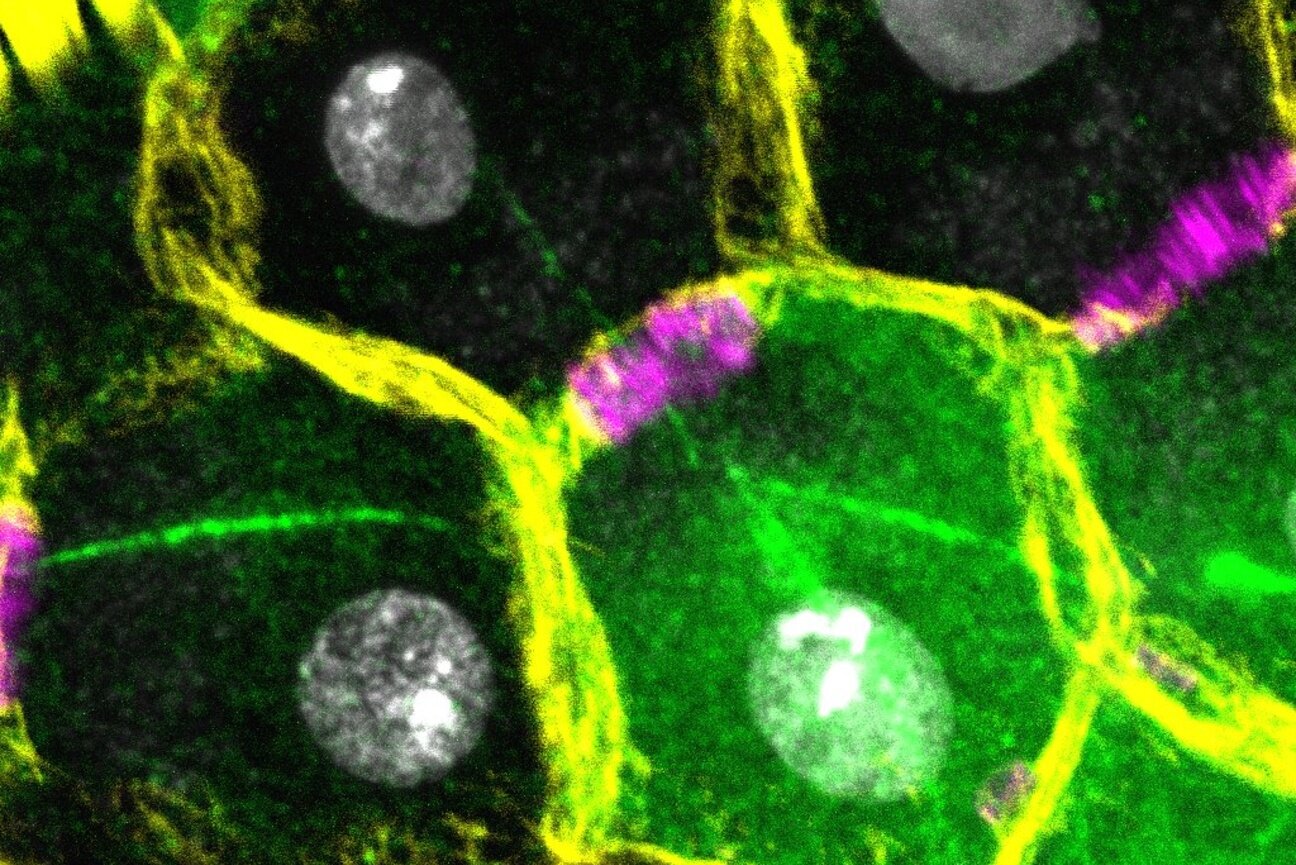
To visualize what was happening, the researchers used immunofluorescence staining (which makes specific proteins glow under a microscope) and FISH—fluorescence in situ hybridization—a method to tag and identify specific chromosomes inside cells.
They found something astonishing: although Ste protein was evenly present in all cells before meiosis, it underwent asymmetric segregation during the first division of meiosis. That is, when the cell split, Ste did not divide equally. Instead, it concentrated in the Y-bearing spermatids, dooming them to a lack of protamines and therefore reproductive failure.
But that still didn’t explain why not all Y-bearing sperm were affected. The answer came in the second round of meiosis. Remarkably, the researchers observed a second asymmetric segregation of the Ste protein. Even if a cell inherited Ste during the first division, only half of its descendants retained it after the second.
The result? Only half of the Y-bearing spermatids were sabotaged.
The Evolutionary Logic of Moderation
This self-limiting behavior is not something anyone expected from a selfish gene. It’s like discovering a con artist who intentionally leaves some of their victims untouched. But in this case, there’s a method to the madness.
By holding back, Ste preserves the production of some Y-bearing sperm. This prevents total collapse of male fertility and allows the population to maintain a roughly balanced sex ratio. It’s a clever evolutionary compromise—maximize transmission without wiping out the host species.
“This self-limiting mechanism is the ultimate solution to the driver-suppressor separation problem,” Yamashita explains. “But the idea is so unconventional that had it been proposed as just a theory, without the evidence we have now, it would’ve been completely dismissed.”
Rethinking Male Meiosis
The implications of this work go far beyond fruit flies. For decades, scientists believed that male meiosis was inherently symmetrical—both daughter cells were treated equally during the divisions that lead to sperm. This study challenges that dogma. If Ste can be unequally distributed, perhaps male meiosis isn’t as symmetrical as we thought.
Is this asymmetry a special trick evolved by Ste to control its own selfishness? Or is it a previously overlooked feature of meiosis that selfish genes have learned to exploit?
The answers to these questions may reshape how we understand inheritance, evolution, and even the very process of life itself.
A New Frontier in Genetics
The discovery of Ste‘s self-limiting behavior is not just a curious exception—it opens up new questions at the heart of evolutionary biology. How many other genes are playing similar games in the germline? Can selfish genes evolve built-in restraints to prevent their own extinction? And can such findings help explain mysterious patterns in sex ratios and fertility across species?
As Yamashita and her team continue their work, the world of genetic inheritance grows ever more intricate—and fascinating.
“The best moments in science are when textbook knowledge is challenged,” says Yamashita, “and it turns out to have been tunnel vision.”
In the intricate chessboard of life’s blueprint, even the genes themselves are strategizing. And sometimes, winning means knowing when not to destroy your opponent.
Reference: Xuefeng Meng et al, Intrinsically weak sex chromosome drive through sequential asymmetric meiosis, Science Advances (2025). DOI: 10.1126/sciadv.adv7089