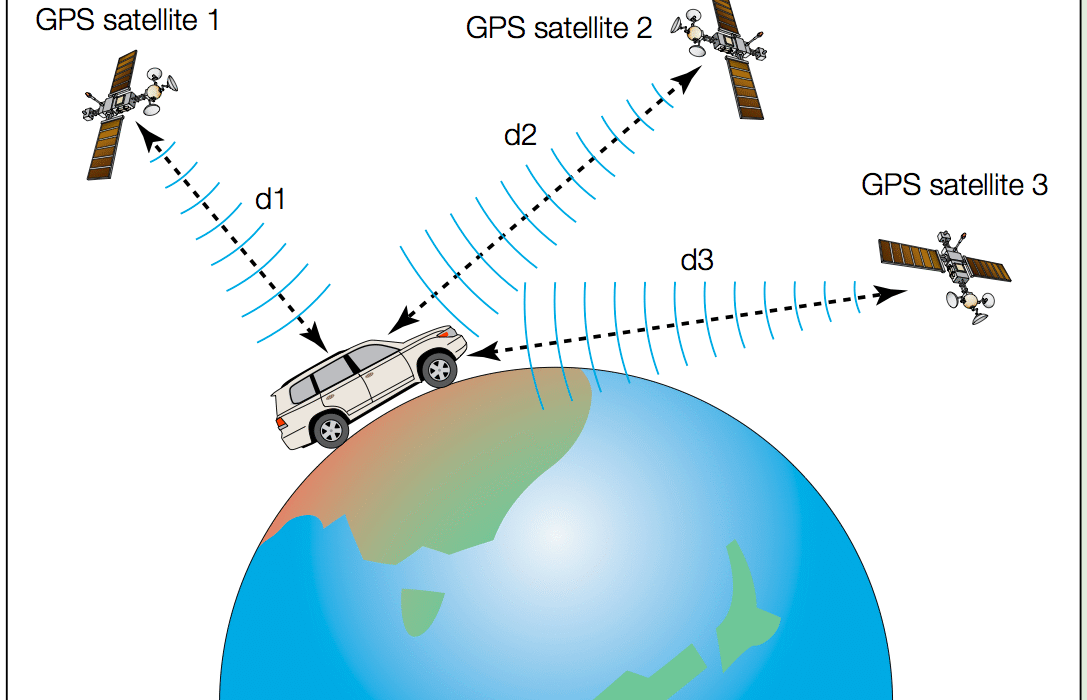
Energy is one of those words we hear daily—from the electricity powering our homes to the food that fuels our bodies. Yet, despite its ubiquitous presence, energy remains a concept that’s often misunderstood or taken for granted. What exactly is energy? Why does it matter? And how does physics explain this mysterious, invisible force that drives the universe?
At its core, energy is the ability to do work or cause change. But this simple definition barely scratches the surface of energy’s profound role in nature. Energy is the thread weaving together the cosmos, the spark igniting life, and the currency of every physical interaction. Understanding energy means understanding how the universe operates—from the tiniest atoms to the vast galaxies—and how humanity harnesses it to shape civilization.
This article dives deep into the physics of energy, unpacking what energy truly is, exploring its different forms, uncovering the laws governing it, and revealing why it matters more than ever in today’s world. Along the way, we will journey through captivating examples, scientific breakthroughs, and real-world implications that highlight energy’s central place in both nature and technology.
What Is Energy? A Fundamental Concept in Physics
In physics, energy is often described as a scalar quantity—meaning it has magnitude but no direction—that quantifies the capacity to perform work. But what does that mean practically? Imagine pushing a box across the floor. The effort you exert transfers energy from your muscles to the box, causing it to move. That transfer of energy doing work on the box is the essence of energy in action.
Energy exists in many forms, all interchangeable but obeying strict physical laws. It can be stored in the chemical bonds of gasoline, carried by photons streaming from the sun, locked inside the nucleus of an atom, or contained as heat in a hot cup of coffee. What they all have in common is the potential or actual ability to produce change—motion, light, heat, or transformation.
Physicists classify energy primarily into two broad categories: kinetic energy and potential energy. Kinetic energy is the energy of motion—whether it’s a speeding bullet, flowing water, or vibrating molecules in a gas. Potential energy, on the other hand, is stored energy, like a drawn bowstring or water held behind a dam, poised to unleash motion or change.
Yet, these categories are just the starting point. When we look closer, energy manifests in many diverse forms, each with fascinating characteristics and roles. Understanding these forms reveals the subtle complexity and beauty of the physical world.
The Many Faces of Energy: Kinetic, Potential, Thermal, and Beyond
Kinetic and potential energy are the familiar pillars, but energy’s full spectrum is vast and varied. Thermal energy, or heat, arises from the random motion of atoms and molecules. The faster these particles move, the higher the temperature and the greater the thermal energy contained within a substance.
Chemical energy is stored within the bonds connecting atoms in molecules. When those bonds break or form during chemical reactions, energy is released or absorbed, powering everything from metabolism in living organisms to combustion engines in cars.
Electrical energy comes from the movement of electric charges, usually electrons flowing through a conductor. It’s the energy behind lightning, batteries, and the electric currents lighting our cities.
Nuclear energy dwells deep within atomic nuclei. It is the energy released when atoms split (fission) or combine (fusion), phenomena that power the sun and nuclear reactors alike.
Radiant energy is carried by electromagnetic waves—light being the most familiar example. This form of energy travels through space, bringing warmth and illumination from the sun and enabling communication via radio waves and X-rays.
Mechanical energy combines kinetic and potential energy associated with objects in motion or positioned within a force field like gravity.
These forms of energy are not isolated but constantly transform from one to another. When you eat food, chemical energy in nutrients converts to kinetic energy as you move and thermal energy to maintain body temperature. When sunlight reaches Earth, radiant energy converts to chemical energy in plants through photosynthesis.
This dynamic interchange underscores one of the most important principles in physics—the conservation of energy.
The Law of Conservation of Energy: Nature’s Unbreakable Rule
One of the bedrocks of physics is the law of conservation of energy, which states that energy cannot be created or destroyed, only transformed from one form to another. This law is fundamental because it tells us that the total amount of energy in an isolated system remains constant.
Imagine a roller coaster at the top of a hill. At that peak, the coaster’s potential energy is at its maximum. As it plunges down the track, that potential energy converts into kinetic energy, speeding the coaster up. At the bottom, its kinetic energy is highest, and potential energy is lowest. If we neglect friction and air resistance, the sum of kinetic and potential energy remains the same throughout the ride. No energy is lost, just exchanged.
This principle has profound implications. It allows physicists and engineers to analyze complex systems, predict outcomes, and design technologies efficiently. From understanding the thermodynamics of engines to the orbits of planets, the conservation of energy is a guiding law.
However, it’s essential to recognize that while energy is conserved, its quality can degrade. For example, when energy converts to heat due to friction, that heat disperses, becoming less available to do useful work. This observation ties closely with the second law of thermodynamics and the concept of entropy.
Entropy and the Second Law of Thermodynamics: The Direction of Energy Flow
While energy cannot be destroyed, the way it spreads and becomes less useful is governed by the second law of thermodynamics. This law states that in any energy transfer, some energy becomes unavailable to do work, usually lost as heat, increasing the system’s entropy—a measure of disorder or randomness.
Entropy explains why natural processes have a preferred direction. A hot cup of coffee left on a table cools down, transferring heat to the surroundings until thermal equilibrium is reached. It never spontaneously heats up by drawing heat from a colder environment. This directional flow of energy from concentrated, useful forms to dispersed, less useful forms is why perpetual motion machines are impossible.
Entropy also underlies the concept of the arrow of time. The universe’s tendency toward increasing disorder means that while physics equations can often run forward or backward, real-world processes have a clear temporal direction—from order to disorder, from energy concentration to dissipation.
This understanding challenges our intuitive notions and has deep consequences in fields ranging from cosmology to biology. It also highlights the importance of energy efficiency and conservation in human technology, as wasted energy ultimately translates to lost resources.
Energy in Motion: The Role of Kinetic Energy
Kinetic energy captures the essence of motion. Any object moving with velocity possesses kinetic energy proportional to its mass and the square of its speed. This relationship, expressed as KE = ½ mv², means that doubling an object’s speed quadruples its kinetic energy, emphasizing how motion dramatically affects energy.
The kinetic energy of particles in gases explains temperature and pressure. Faster-moving molecules collide more energetically, raising temperature and exerting greater pressure. This microscopic kinetic energy manifests as thermal energy we can feel.
In the macroscopic world, kinetic energy drives transportation, from bicycles to rockets. It also plays a crucial role in collisions, impacting forces experienced by objects and structures. Engineers must account for kinetic energy to design safer vehicles and buildings.
Beyond classical objects, kinetic energy extends to waves and fields. Electrons moving in a conductor carry kinetic energy that powers electrical devices. Photons, though massless, carry energy related to their frequency, behaving like particles in the quantum realm.
Understanding kinetic energy bridges everyday experiences and fundamental physics, illustrating energy’s dynamic nature.
Potential Energy: The Power of Position and Configuration
Potential energy is stored energy resulting from an object’s position or arrangement relative to a force. Gravity and springs are classic examples. An apple held above the ground contains gravitational potential energy because gravity can pull it down, converting stored energy into motion.
Elastic potential energy exists in stretched or compressed springs. When released, this energy transforms into kinetic energy, causing movement. Potential energy can also be electrical, arising from charges separated by a distance, or chemical, embedded in molecular structures.
Potential energy’s beauty lies in its latent power—a silent promise of change. It shapes the orbits of planets, the mechanics of machines, and even biochemical reactions inside cells.
Thermal Energy and Heat: The Microscopic Dance
Thermal energy arises from the random motion of atoms and molecules. Temperature measures the average kinetic energy of these particles. When thermal energy transfers between systems, we perceive it as heat.
Heat flows naturally from hotter to colder bodies until equilibrium is reached, driven by the second law of thermodynamics. This transfer can occur via conduction, convection, or radiation.
Thermal energy powers steam engines, warms our homes, and influences weather patterns. At a microscopic level, it plays a role in phase changes, like melting and boiling, as energy breaks or forms bonds between molecules.
Understanding thermal energy is essential for improving energy efficiency and developing technologies such as heat engines, refrigerators, and even climate models.
Chemical Energy: Nature’s Fuel
Chemical energy resides in the bonds holding atoms together in molecules. Breaking or forming these bonds during chemical reactions releases or absorbs energy. This process fuels life, powers vehicles, and drives industry.
Photosynthesis converts solar energy into chemical energy stored in plants. Animals consume plants, transforming chemical energy into motion and heat. Fossil fuels are ancient chemical energy reserves formed from decomposed organic matter.
Chemical energy’s importance transcends biology and technology, influencing everything from metabolism to global energy markets.
Nuclear Energy: Unlocking the Atom’s Heart
Nuclear energy stems from forces within atomic nuclei. Fission splits heavy atoms like uranium, releasing enormous energy harnessed in nuclear reactors and weapons. Fusion combines light nuclei, powering the sun and promising future clean energy sources.
Nuclear processes challenge our understanding of energy scales and fundamental forces. They also pose ethical and environmental questions about harnessing such powerful energy.
Electromagnetic Energy: Light and Beyond
Electromagnetic energy travels as waves of electric and magnetic fields. Visible light is just a small part of the electromagnetic spectrum, which includes radio waves, microwaves, X-rays, and gamma rays.
Photons, particles of light, carry energy proportional to their frequency. Electromagnetic energy enables vision, wireless communication, and medical imaging.
Harnessing electromagnetic energy has revolutionized modern life and continues to drive technological innovation.
Energy Transformations and Conservation in Everyday Life
Energy rarely stays in one form. It continually transforms—chemical to kinetic in a car engine, electrical to radiant in a light bulb, or nuclear to thermal in a power plant. These transformations are never perfectly efficient, always governed by thermodynamic laws.
Understanding these conversions helps optimize technologies, reduce waste, and develop sustainable energy solutions.
Why Energy Matters: Practical and Philosophical Perspectives
Energy’s importance extends beyond physics into daily life, economics, environment, and ethics. It underpins industry, transportation, communication, and healthcare. Our civilization’s progress depends on accessing and managing energy resources wisely.
Philosophically, energy connects us to the universe’s fundamental workings. It invites reflection on humanity’s place in nature and responsibilities toward future generations.
The Future of Energy: Challenges and Innovations
Facing climate change and resource depletion, humanity stands at a crossroads in energy use. Transitioning to renewable sources like solar, wind, and fusion is crucial for sustainability.
Emerging technologies in energy storage, smart grids, and efficiency promise to reshape our energy landscape. Understanding energy physics equips us to meet these challenges.
Conclusion: Embracing Energy’s Central Role
Energy is more than a scientific concept; it is the essence of change, motion, and life itself. Grasping the physics of energy enriches our appreciation of nature and empowers us to innovate responsibly.
By exploring energy’s forms, laws, and impacts, we unlock insights vital to science, technology, and society. In a world powered by energy, understanding it matters—deeply and urgently.