In the mysterious, muscular corridors of the human gut, an extraordinary ballet unfolds every moment of our lives. With wave-like contractions, known as peristalsis, our intestines rhythmically churn food, extract nutrients, and move waste down the line. This process seems almost effortless—until it goes wrong. Disorders of gut motility can wreak havoc on health, from chronic constipation to life-threatening neonatal conditions. And yet, one question has eluded scientists for decades: How do intestinal muscle cells know when to contract?
Now, a groundbreaking study from researchers at UC Davis and UCLA has revealed a crucial part of the answer. A pressure-sensing protein called Piezo1, once thought to operate only on the cell surface, appears to act as an internal motion sensor deep inside gut smooth muscle cells. It doesn’t just respond to mechanical force—it helps orchestrate the entire muscular symphony that drives digestion.
The findings, published in Communications Biology, open a new window into how our bodies regulate intestinal motion—and may pave the way for transformative treatments for complex gut disorders.
A Muscle That Thinks with Pressure
Unlike skeletal muscle, which responds to voluntary signals from the brain, smooth muscle in the intestines operates almost autonomously. It pulses and flexes in tune with subtle cues—many of them mechanical rather than neural. Until recently, scientists assumed that stretch or pressure in the gut wall activated surface receptors, which in turn triggered contraction through calcium signaling. But that model left many gaps.
Enter Piezo1, a mechanically activated ion channel already known for its role in sensing blood pressure and regulating vascular tone. The protein had never been considered a major player in gut physiology—until now.
The UC Davis-UCLA team, led by neonatologist Dr. Geoanna Bautista and pediatric gastroenterologist Dr. Martín G. Martín, took a deep dive into Piezo1’s role in the digestive system using genetically engineered mice. What they discovered was not just surprising—it was revolutionary.
What Happens When Piezo1 Goes Missing?
To test Piezo1’s function, researchers used a knockout model where the gene encoding Piezo1 was removed specifically from smooth muscle cells in the gut. What happened next stunned them. The mice experienced significant weight loss, food transit delays, and thinning of the intestinal muscle layers—classic signs of impaired gut motility.
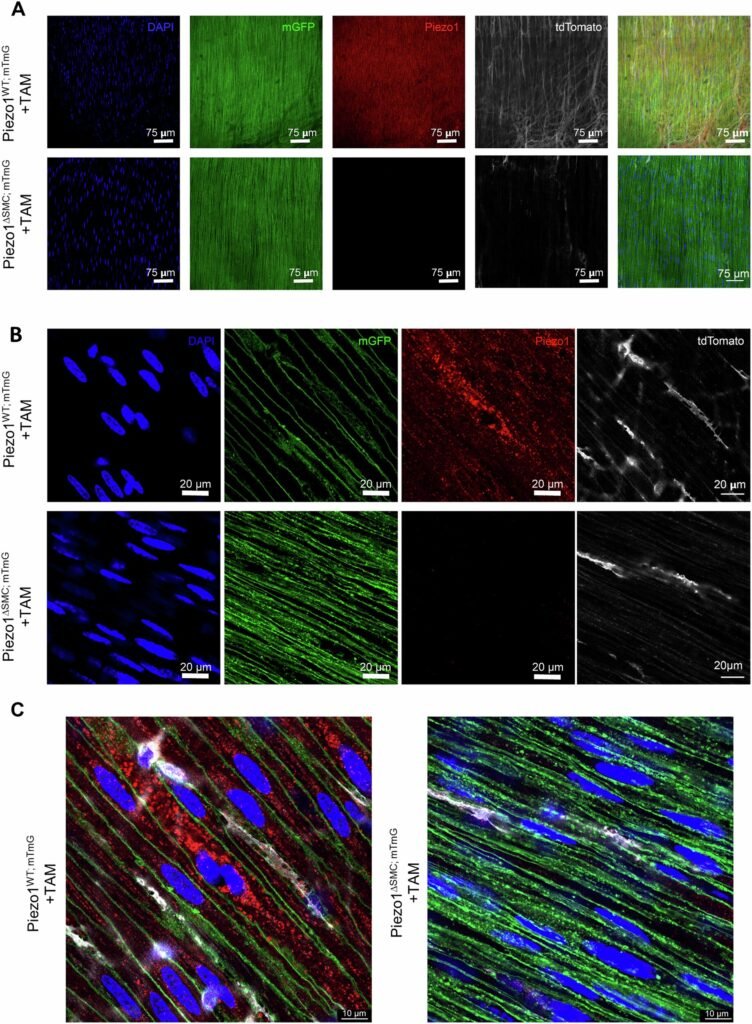
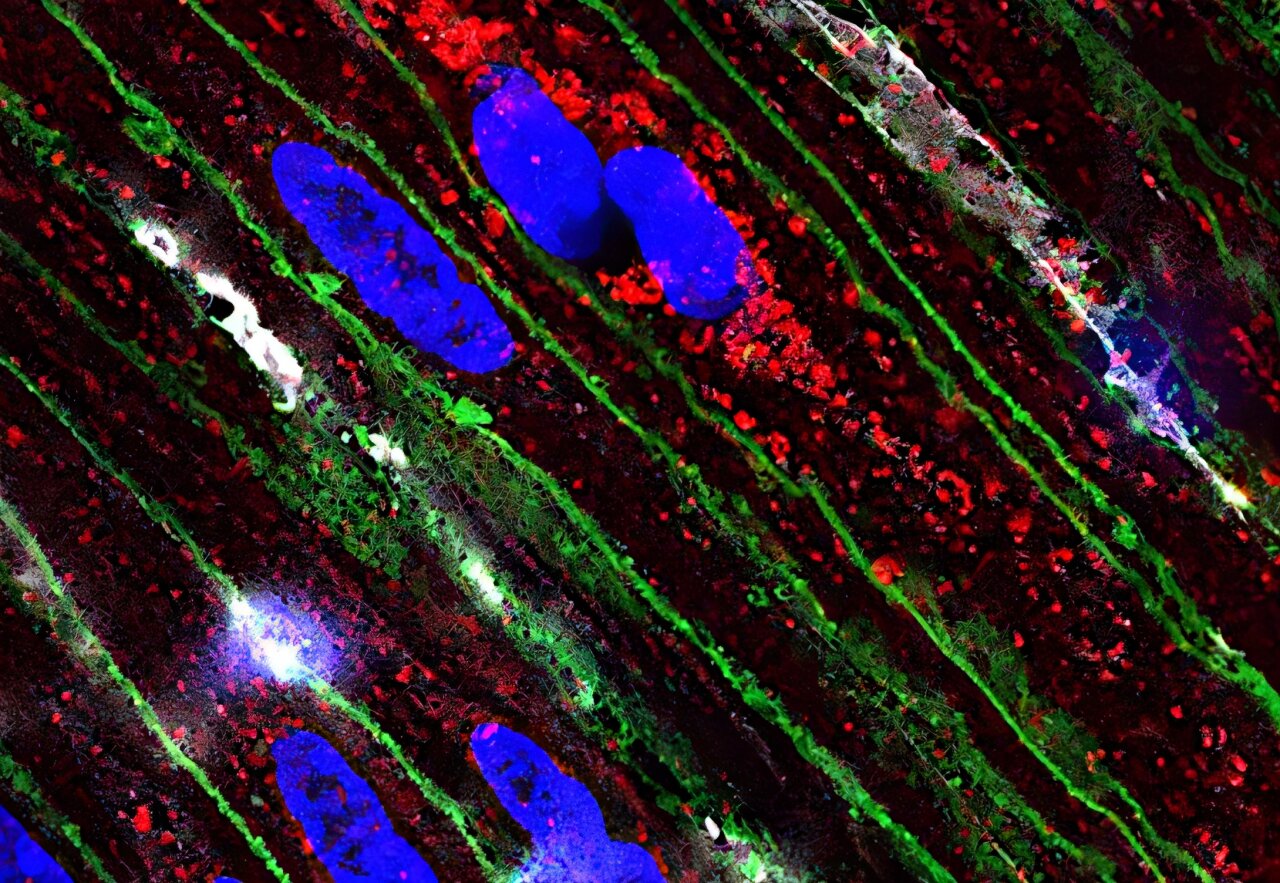
At the microscopic level, the absence of Piezo1 disrupted a finely tuned biochemical ballet. Calcium ions, the critical messengers that cue muscle fibers to contract, failed to move properly. This interference wasn’t just a mechanical issue; it was a failure in the gut’s fundamental communication system.
“These mice showed profound physiological changes, yet remarkably, they lived as long as normal mice,” said Dr. Bautista. “It speaks volumes about the adaptability of the gut and how layered its regulatory systems truly are.”
A Surprise Inside the Cell
Perhaps the most unexpected finding came not from what Piezo1 was doing, but where it was doing it.
“We always thought of Piezo1 as sitting on the cell membrane, detecting stretch and pressure from the outside,” explained Dr. Martín. “But in these smooth muscle cells, we found Piezo1 tucked inside—within the sarcoplasmic reticulum,” the muscle’s internal calcium storehouse.
This intracellular placement suggests that Piezo1 is not merely a mechanical gatekeeper—it may be a master regulator of internal calcium signaling, responsible for timing and modulating the strength of muscle contractions. Even when conventional calcium channels were blocked, Piezo1 offered an alternative pathway to sustain some level of muscular rhythm.
This mechanism may explain why the gut’s motion persists, albeit dysfunctionally, even when traditional signaling fails—a clue that could help clinicians understand and eventually correct disorders where gut motility goes haywire.
When the Gut’s Rhythms Go Wrong
Gut motility disorders—conditions in which the intestines fail to contract properly—are notoriously hard to treat. Patients suffer from chronic discomfort, malabsorption, or even complete digestive failure. In newborns, especially preterm infants, these conditions can be fatal. Diseases like necrotizing enterocolitis (NEC), gastroschisis, and short bowel syndrome present devastating clinical challenges.
Understanding the role of Piezo1 could dramatically shift this landscape. As Dr. Bautista’s team discovered, when Piezo1 was absent, the gut tried to compensate by ramping up other calcium-related proteins—but it wasn’t enough. This suggests Piezo1 doesn’t just participate in gut contraction; it may be a primary regulator, orchestrating multiple downstream pathways essential for proper digestion.
“This gives us a completely new perspective,” Bautista said. “Piezo1 may be a previously unrecognized cornerstone of gut health.”
The Fetal Frontier
As exciting as these findings are, they may just be the beginning. Dr. Bautista is now focusing her research on fetal gut development, hoping to understand how Piezo1 shapes the digestive system even before birth.
In premature infants, the gut often struggles to function outside the womb. If Piezo1 plays a pivotal role in fetal gut motility, its dysfunction or absence might underlie a wide range of postnatal conditions. This could lead to early diagnostic tools or even prenatal interventions for babies at risk of severe gastrointestinal complications.
“We’re looking at Piezo1 as not just a pressure sensor but a developmental signal,” Bautista said. “It may help guide how the gut forms and learns to move.”
A Potential Therapeutic Target
Piezo1 is more than a scientific curiosity. Because it functions as an ion channel, it’s inherently druggable—a potential target for small molecules that could enhance or mimic its activity. In theory, activators of Piezo1 could one day help restore gut motility in patients suffering from paralyzed or sluggish intestines.
On the flip side, overactive Piezo1 signaling might contribute to conditions like irritable bowel syndrome (IBS) or intestinal spasms. If so, Piezo1 blockers could be equally valuable in calming the gut’s overzealous movements.
In this way, Piezo1 presents not just a diagnostic clue, but a therapeutic lever—a new tool in the fight against gastrointestinal disease.
A Broader Biological Role?
Beyond the gut, Piezo1 is turning out to be a molecular multitasker. It’s already implicated in red blood cell volume regulation, vascular development, and bone formation. Its ability to transduce mechanical force into biological signals hints at a fundamental role in how the body senses its own shape and movement.
In the context of digestion, Piezo1 may serve as a kind of internal proprioceptor, helping the gut “feel” itself—its fullness, its stretch, its resistance. That ability may be especially important during moments of mechanical stress, such as swallowing large food particles or navigating blockages.
In the ever-churning machinery of the digestive system, Piezo1 may be the silent conductor, ensuring that each muscular contraction is perfectly timed, precisely calibrated, and effortlessly coordinated.
The Gut’s Watchful Guardian
The gut has long been called the “second brain,” a hub of neurological and immunological activity with direct influence on our mood, metabolism, and immunity. Now, with Piezo1 in the picture, we may need to add another dimension to our understanding—a mechanical one.
By translating physical pressure into cellular action, Piezo1 bridges the gap between the gut’s form and its function. It ensures that our digestive tract doesn’t just sit and wait, but actively responds, adapts, and evolves in response to the pressures of life—quite literally.
As the science advances, we may one day learn to harness this tiny protein’s power to heal diseased intestines, restore broken rhythms, and support premature infants whose digestive systems have not yet learned the music of motion.
For now, Piezo1 stands as a compelling reminder that some of biology’s greatest insights lie not in vast organs or sprawling genomes, but in the smallest, most unexpected places—deep inside a single cell, quietly tuning the rhythms that keep us alive.
Reference: Geoanna M. Bautista et al, Smooth muscle cell Piezo1 depletion results in impaired contractile properties in murine small bowel, Communications Biology (2025). DOI: 10.1038/s42003-025-07697-6