For decades, the spleen has been the underdog of the human body—an organ often regarded as expendable, overshadowed by its more glamorous neighbors like the liver, pancreas, and kidneys. But recent groundbreaking research from Wenzhou Medical University is upending that notion. Scientists there have reimagined the spleen not as an afterthought, but as a sophisticated host for a medical revolution: long-term blood sugar control in diabetes, powered by islet transplantation without the immune-suppressive baggage that typically follows.
The findings, published in Science Translational Medicine, mark a bold departure from traditional approaches to managing type 1 diabetes. By using nanoparticles to re-engineer the spleen’s immune landscape and vascular architecture, researchers successfully transformed it into a welcoming habitat for transplanted islet cells—those delicate pancreatic clusters that produce insulin.
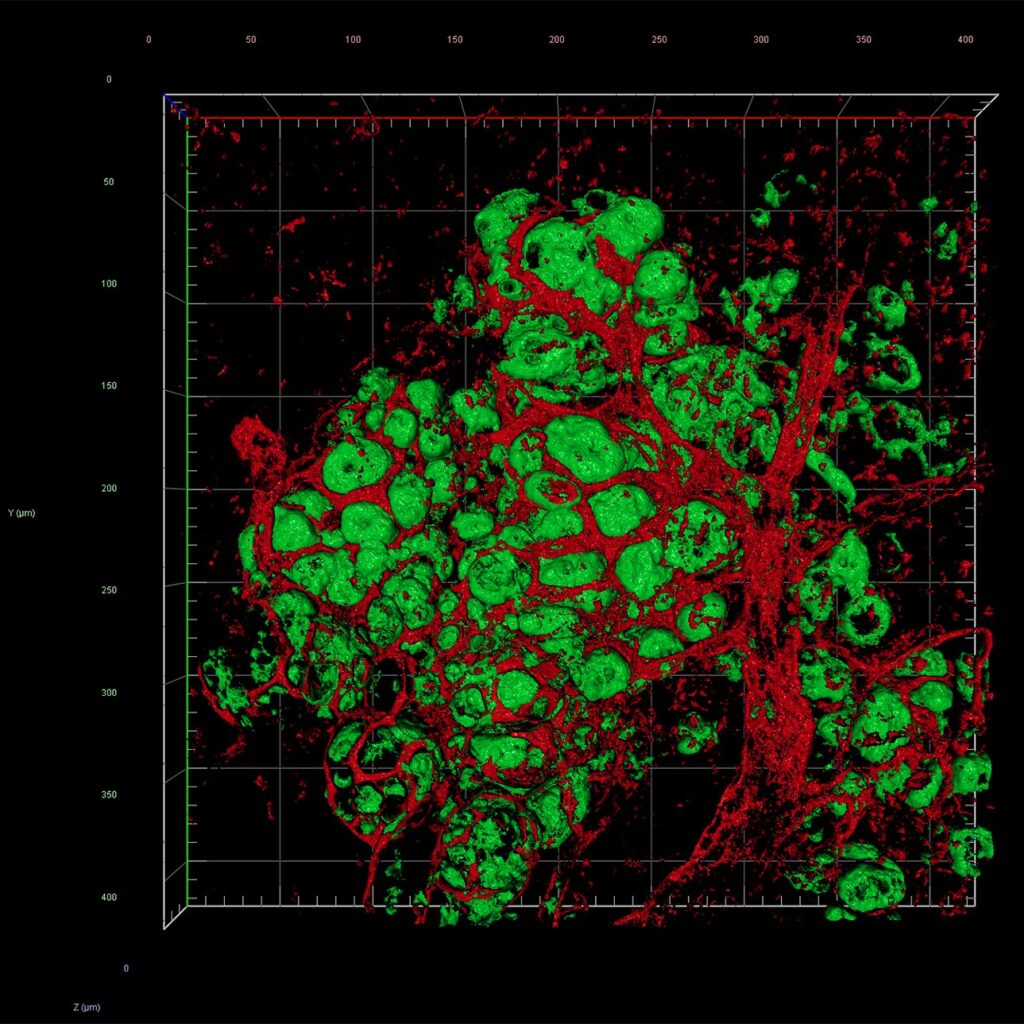
In both mice and nonhuman primates, transplanted islets not only survived, but thrived in their remodeled splenic sanctuary. They restored normal blood glucose levels, sustained insulin production, and avoided the devastating immune attack that typically dooms such grafts. The spleen, once overlooked, is now poised to become an unlikely hero in the fight against diabetes.
The Central Problem in Type 1 Diabetes
Type 1 diabetes is an autoimmune disorder in which the body’s immune system mistakenly attacks and destroys its own beta cells—the insulin-producing cells nestled in the islets of Langerhans within the pancreas. Without insulin, cells can’t absorb glucose from the bloodstream, leading to dangerously high blood sugar levels that, if left untreated, cause severe organ damage and death.
The mainstay of treatment has long been insulin injections, but this approach is a daily balancing act, requiring constant vigilance and fine-tuned dosing. Even with modern insulin pumps and continuous glucose monitors, maintaining perfect control is elusive for most patients. That’s where islet transplantation comes in.
This technique, developed in the late 20th century, involves transferring islets from a donor pancreas into the recipient’s liver via the portal vein. The hope is that these transplanted cells will take up residence and resume natural insulin production, freeing patients from daily injections. But the liver, it turns out, is a hostile landlord.
Why the Liver Falls Short
Islet transplantation to the liver has always been a paradox: it’s convenient, but not particularly kind to its new residents. The hepatic microenvironment, where the islets are deposited, is rife with threats. Within hours of transplantation, up to 70% of islet cells can be destroyed due to a perfect storm of immune attack, low oxygen levels, and physical stress from the liver’s dense, inflexible tissue. This devastating attrition means that many recipients need islets from multiple donors—a severe limitation in a field already grappling with donor shortages.
Over the years, researchers have scouted alternative transplant sites, from the eye and muscle tissue to the fatty folds of the omentum. But these alternatives often demand invasive surgery, offer little improvement in islet survival, or distort the natural timing and feedback loops of insulin secretion. The search for a truly optimal site remained frustratingly elusive—until the spleen stepped into the spotlight.
A High-Tech Makeover for the Spleen
The innovation from Wenzhou Medical University centers on a simple but powerful idea: what if you could precondition the spleen to accept and protect islet grafts by reshaping its immune environment and vascular structure?
To do this, the researchers turned to a clever tool: biodegradable silica nanoparticles coated with konjac glucomannan, a natural compound derived from the root of the konjac plant. These tiny particles were injected directly into the spleens of both diabetic rodents and cynomolgus macaques. Over the course of several weeks, the nanoparticles worked like molecular architects, remodeling the splenic tissue.
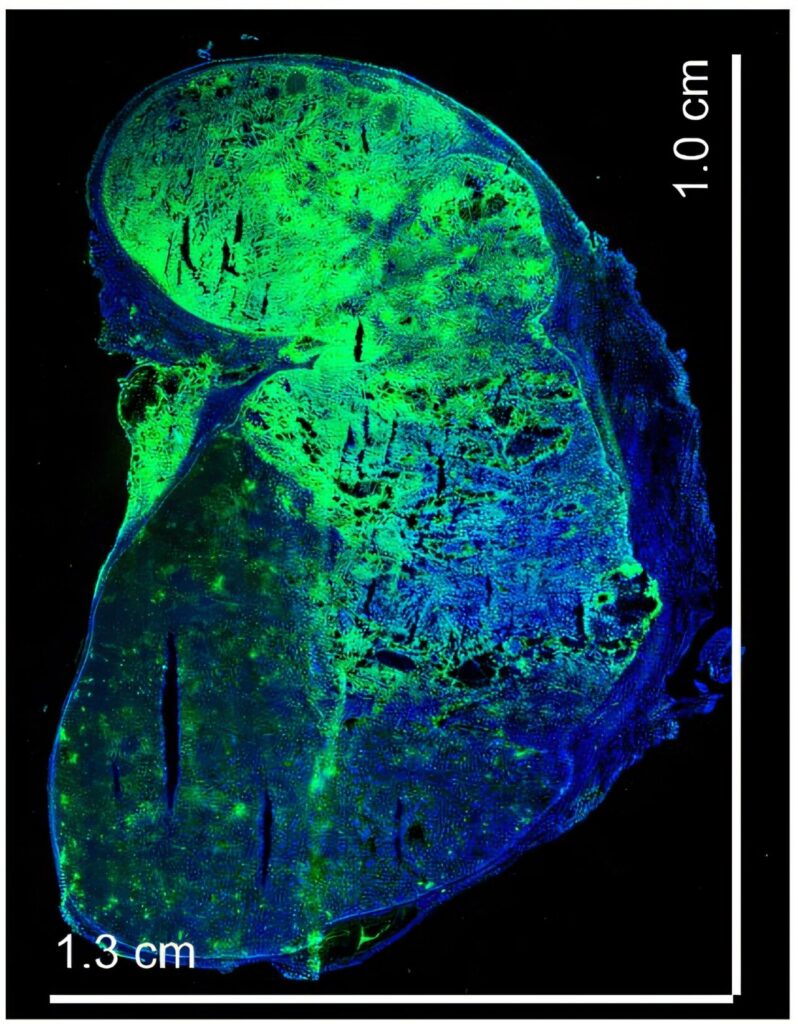
In mice, this involved four injections over two weeks, after surgically repositioning the spleen outside the peritoneal cavity for easier access and observation. In macaques, the process was more sophisticated: ultrasound-guided injections delivered the nanoparticles into the upper, middle, and lower regions of the spleen over four weeks.
The result? A remarkable transformation. The spleen became more vascularized and immunologically tolerant—essentially turning into a specialized organ tailor-made for islet graft survival.
A Cellular Refuge: How the Spleen Shields Islets
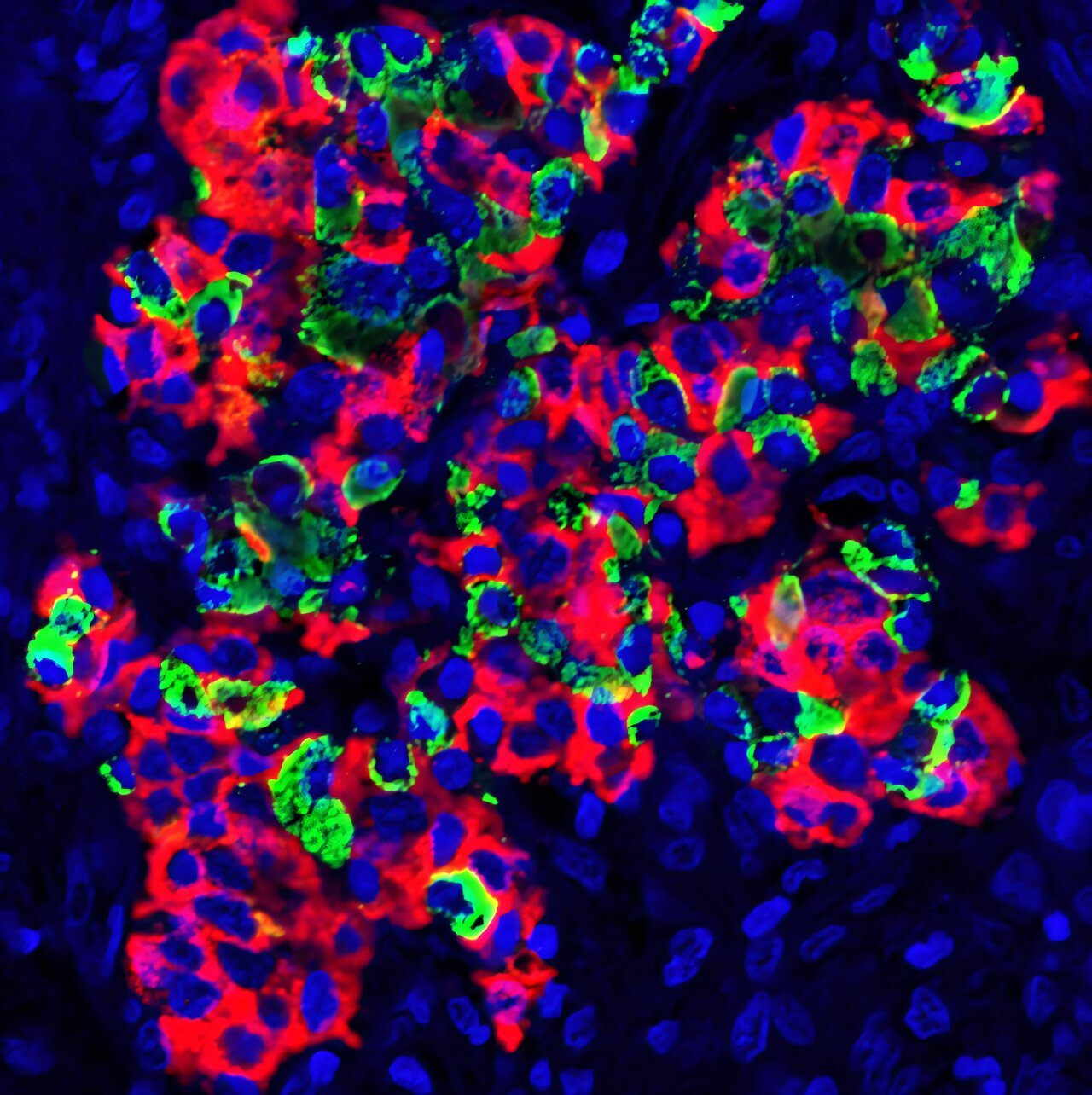
To test the strategy, the team transplanted mouse and rat islets into remodeled spleens of diabetic mice and human islets into the spleens of macaques. In all cases, the donor islets were prepared using collagenase digestion and density-gradient purification—standard techniques in islet isolation.
The outcomes were nothing short of astonishing.
In mice, islet grafts survived for at least 90 days, maintaining their architecture and function without signs of immune rejection. In control animals, where spleens were not preconditioned, the islets were swiftly destroyed within a week. The contrast couldn’t be clearer.
Within days of transplantation, blood vessels grew into the grafts. By two weeks, the islets were embedded in a dense network of capillaries, ensuring a rich oxygen and nutrient supply—an essential factor in their survival and function. In macaques, human islets survived and remained functionally active for at least 28 days, with strong evidence of revascularization and insulin secretion, even under reduced immunosuppression.
Immune Alchemy: Turning Foes into Friends
The spleen’s new tolerance wasn’t just about better blood flow. It was immunological magic.
Detailed analysis revealed a dramatic shift in the local immune environment. The injected nanoparticles coaxed the spleen into generating more regulatory T cells—immune sentinels that prevent autoimmunity—and M2 macrophages, a type of immune cell known for tissue repair and tolerance. Simultaneously, effector T cells (the ones responsible for aggressive immune attacks) were suppressed, and the production of inflammatory cytokines dropped.
In short, the immune system, rather than launching an all-out assault on the graft, stood down.
Even in the macaques, where human islets represented a more dramatic cross-species transplant, the immune system reacted gently. Expression profiles showed a marked rise in anti-inflammatory genes and a calming of T cell activation. In vitro tests confirmed that the nanoparticles dampened T cell proliferation and nudged macrophages into their regulatory, healing mode.
From Bench to Bedside: The Promise and the Path Forward
The most exciting part? It worked.
In mice, the transplanted islets rapidly restored blood sugar control. Glucose levels normalized within days and stayed stable for three months. Glucose tolerance tests and insulin assays revealed profiles almost identical to healthy mice.
In macaques, human islets maintained insulin and C-peptide secretion for nearly a month—an unprecedented result under limited immunosuppression. When researchers performed splenectomy (spleen removal), glycemic control abruptly deteriorated, proving the grafts were responsible for the restored function.
This experimental success opens a tantalizing path toward clinical application. If similar results can be replicated in humans, this could transform islet transplantation from a risky, donor-intensive, liver-based procedure into a spleen-based therapy that is more reliable, less invasive, and dramatically more effective.
Why the Spleen Matters Now More Than Ever
Beyond the stunning results, this study marks a conceptual shift. It suggests that instead of fighting the immune system with brute-force immunosuppressants, we can train it—coax it—into tolerance through precise engineering of the microenvironment.
This idea of “organ reprogramming” opens up exciting possibilities not just for diabetes, but for other conditions where cell transplantation might be beneficial—such as Parkinson’s disease, hemophilia, or even cardiac repair. The spleen, with its dual role in blood filtration and immune education, may prove to be a uniquely adaptable platform for future regenerative medicine.
For type 1 diabetes, the implications are immediate and profound. If islet transplantation can be made more efficient, more durable, and less dependent on immunosuppression, it could evolve from an experimental therapy into a standard of care. It could free patients from insulin dependency, flatten the rollercoaster of blood sugar swings, and dramatically reduce the long-term complications of diabetes.
A Revolution Begins, Quietly
In science, breakthroughs often arrive not with a bang, but with the quiet rustle of a new idea taking root. In this case, it was not a flashy technology or a miracle drug, but a shift in where we look—a willingness to see the spleen not as disposable, but as indispensable.
Through an elegant dance of biology, nanotechnology, and immunology, Wenzhou Medical University’s researchers have transformed an old, overlooked organ into the cornerstone of a bold new therapy. Their success represents not just a triumph in diabetes research, but a poetic reversal of expectations: the spleen, once forgotten, may now help rewrite the future of medicine.
And in that transformed tissue, in those thriving islets, lies the possibility that millions with diabetes might one day live not with management, but with a cure.
Reference: Mi Liu et al, Islet transplantation in immunomodulatory nanoparticle–remodeled spleens, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adj9615