In the smoldering cradle of early Earth, long before forests danced in the breeze and whales sang across oceans, life flickered into being in the darkness. It was a world without oxygen, without plants, without even a proper sunlit sky. And yet, somewhere amid the boiling waters of deep-sea vents, the very first cells sparked into existence—simple, robust, and hungry for energy.
Now, over 4 billion years later, scientists at Ludwig-Maximilians-Universität (LMU) Munich have reached back into that abyss of time and reawakened the ancient metabolism that may have powered those earliest living organisms. Their experiment doesn’t just simulate the origin of life—it resuscitates it.
The Forgotten Ancestors and Their Fiery World
The earliest known ancestor of all life on Earth—our microbial great-grandparent—likely resembled a tiny heat-loving archaeon that thrived not on sugar or oxygen, but on hydrogen gas. This primal cell drew energy from the planet itself, converting hydrogen and carbon into methane through a metabolic pathway known as the acetyl-CoA pathway.
Today, this pathway still pulses through the biochemistry of many archaea and bacteria, considered by many biologists to be one of the most ancient and fundamental metabolic circuits in all of biology. But until now, scientists could only study its present-day manifestations. The original chemical conditions that sparked such a process—what might have breathed energy into Earth’s first cells—had remained out of reach.
That is, until Professor William Orsi and his team at LMU decided to rebuild the lost world.
Rebuilding a Primordial Earth in Glass and Steel
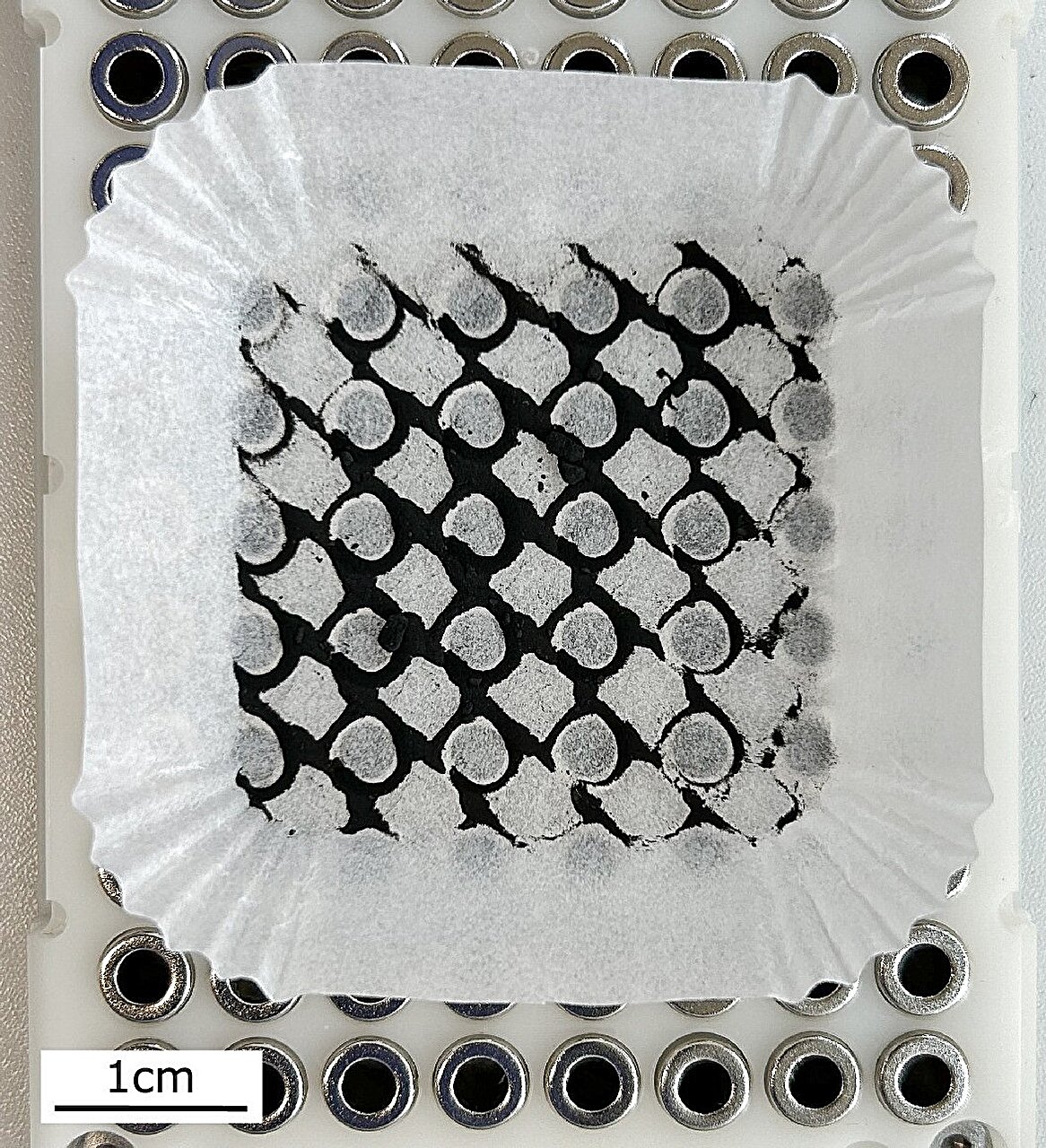
Imagine peering into a time capsule not made of words or images, but of chemical gradients and mineral formation. That’s essentially what Orsi’s team did: they constructed “chemical gardens”—laboratory simulations of hydrothermal vents from 4 to 3.6 billion years ago.
These artificial vents mirrored the rugged terrain of Earth’s early seafloor, the kind of volcanic, mineral-rich environment where life might have first emerged. Known today as black smokers, these submarine geysers exhale mineral-laden plumes into the ocean and are still home to strange and resilient organisms. The early Earth versions, however, had a major twist—an ocean saturated with dissolved iron, vastly different from the oxygenated waters of today.
Within this iron-rich broth, the researchers triggered abiotic reactions—that is, reactions not caused by life—between iron and sulfur, mimicking the geological forces that once sculpted Earth’s crust. These reactions produced iron sulfide minerals, particularly mackinawite (FeS) and greigite (Fe₃S₄). Alongside these minerals, the reactions released hydrogen gas (H₂)—a crucial, high-energy molecule thought to have been the very first fuel for life.
In this geochemically active setting, the team introduced a living relic of the past: Methanocaldococcus jannaschii, an extremophile archaeon that still relies on hydrogen to survive, just as its ancestors likely did billions of years ago.
A Surprising Bloom in a Garden of Stone
The outcome of the experiment was nothing short of astonishing. The researchers had expected minimal growth—after all, no additional nutrients, no vitamins, not even trace metals had been added to help the cells along. This was to be a trial by fire, a return to nature red in tooth and claw. But Methanocaldococcus jannaschii didn’t just survive—it flourished.
“We were shocked to see exponential growth,” said Vanessa Helmbrecht, the study’s lead author. “It was thriving purely on the hydrogen gas produced by these geochemical reactions. That’s energy being generated not by life, but by the Earth itself.”
Moreover, the organism upregulated key genes associated with the acetyl-CoA pathway, as if recognizing the familiar energy-rich conditions of its ancient evolutionary past. It responded not with dormancy or distress, but with vigorous, self-sustaining life.
The cells even gravitated toward the iron sulfide minerals, growing in close proximity to mackinawite particles—echoing fossil evidence of some of the earliest microbial mats, where mineral imprints in ancient rocks still preserve the ghostly outlines of life.
The Metabolism That Preceded All Others
What the LMU team accomplished wasn’t just a clever lab trick. They provided the most direct demonstration yet that life could have begun not from complex molecules or divine intervention, but from simple geochemistry.
Hydrogen-dependent methanogenesis via the acetyl-CoA pathway is now seen as a biochemical time capsule, a system of energy production so ancient that it predates photosynthesis, oxygen respiration, and nearly all other known forms of metabolism. This process may very well represent the oldest mechanism by which life extracted usable energy from its environment.
This finding aligns with a growing body of evidence suggesting that the origin of life was a geological inevitability, given the right conditions. Life, in this view, didn’t erupt as a freak accident but slowly crystallized from the intimate dance of rock, water, and heat.
Black Smokers and the Birth of Cells
The structures recreated in LMU’s lab are analogs of hydrothermal vents—vast undersea formations where magma meets water, triggering a kaleidoscope of chemical reactions. These are not peaceful environments. They’re pressure-cooked, metal-rich crucibles capable of sustaining 300°C temperatures.
Yet, amid this hellish beauty, life appears to thrive. And it may have done so from the very beginning.
The idea is not new. Decades ago, biologist Günter Wächtershäuser proposed that life began on the surfaces of iron sulfide minerals, catalyzing carbon reactions that gradually became self-replicating. LMU’s findings represent one of the strongest experimental validations of that hypothesis to date.
Peering Beyond Earth: Could Life Elsewhere Be Born the Same Way?
The implications of the study extend far beyond our own world. If life can begin this way—through rock-water chemistry, fueled by planetary heat—then perhaps it doesn’t require a lush Earth at all.
Professor Orsi and his team are now setting their sights on Saturn’s moon Enceladus, a gleaming ice sphere that may hide hydrothermal vents in a salty ocean beneath its frozen shell. NASA’s Cassini spacecraft has already detected plumes of hydrogen gas venting from Enceladus’s surface—a potential sign of deep-sea geochemistry not unlike early Earth’s.
“We’re going to simulate Enceladus conditions in the lab,” Helmbrecht explained. “We want to know if organisms like Methanocaldococcus jannaschii could survive and grow there too.”
If they can, it would be a profound shift in our search for extraterrestrial life. We’d no longer be looking only for planets like Earth, but for planets—or moons—with iron, sulfur, water, and heat.
A Humble Microbe With Galactic Implications
Methanocaldococcus jannaschii may seem insignificant: a microbe that lives in boiling water and consumes hydrogen. But this tiny archaeon is a time traveler of sorts, still whispering the story of how all life—trees, birds, whales, and humans—originated from the elemental Earth.
Its success in the LMU laboratory confirms that life’s earliest metabolism is not only feasible, but perhaps inevitable wherever the right chemistry brews. This insight collapses the distance between life and non-life, suggesting that the transition may be much simpler—and much more common—than once thought.
Science Reimagines the First Spark
The LMU researchers have achieved more than a brilliant scientific feat—they’ve touched the edge of a mystery that has haunted human thought for centuries: How did life begin?
Not with lightning bolts and magical soups, as old theories posited, but with iron, heat, and hydrogen, stirred in the dark belly of the planet. No chlorophyll. No mitochondria. Just chemistry, rolling slowly forward, until the first cell caught fire.
And now, in a modern lab under sterile lights, we see that fire again.
Reference: Vanessa Helmbrecht et al, Simulated early Earth geochemistry fuels a hydrogen-dependent primordial metabolism, Nature Ecology & Evolution (2025). DOI: 10.1038/s41559-025-02676-w