Floating high above Venus’s scorched and seething surface lies a realm that has long fascinated scientists—a perpetual shroud of thick, acidic clouds. For decades, these skies were deemed hellish and incompatible with life: a brew of sulfuric acid droplets, chlorine, iron, and crushing atmospheric pressures, all under blistering heat. Surely, no molecule resembling the fragile biochemistry of Earth could survive such brutality. Yet a new study is challenging that assumption—and opening a thrilling new chapter in the search for life beyond Earth.
Researchers led by Dr. Janusz Jurand Petkowski from Wrocław University of Science and Technology have revealed that peptide nucleic acid (PNA)—a synthetic cousin of DNA—can survive under laboratory conditions designed to mimic the ferocious atmosphere of Venus’s clouds. Published in Science Advances, this groundbreaking study doesn’t just broaden our ideas of habitability—it redefines the very chemistry of life.
Redefining the Limits of Life
When astrobiologists ponder the question of extraterrestrial life, they often look for the familiar: water-based chemistry, Earth-like temperatures, oxygen, and carbon-based biomolecules. Venus, Earth’s twin in size but not in temperament, has largely been cast aside in this search. Its surface temperature hovers around 470°C—hot enough to melt lead—and its clouds are composed of roughly 98% sulfuric acid, one of the most corrosive substances known.
Yet these clouds, located 40 to 60 kilometers above the planet’s surface, maintain a surprisingly moderate range of temperatures—from 0°C to 100°C. This relative temperance led scientists to wonder: could something survive here? Not life as we know it, but a form based on alien chemistry? That’s the question that the international team set out to explore—with PNA at the center of their inquiry.
PNA: A Molecular Impostor with Hidden Powers
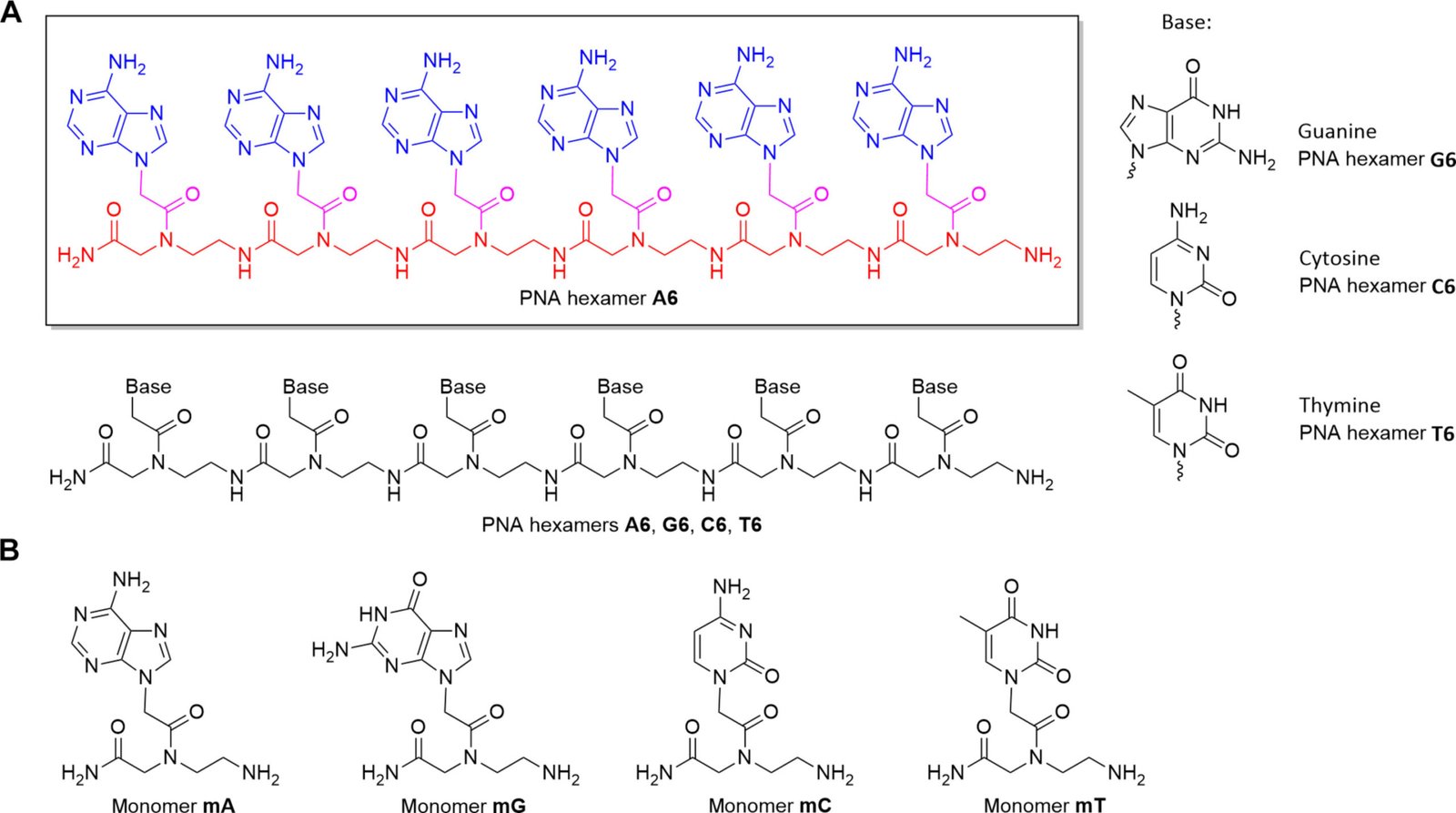
Peptide nucleic acid is not found in nature, at least not on Earth. It was first synthesized in the 1990s as a more chemically stable analog of DNA. Like DNA, it can encode information, bind to complementary sequences, and form double helices. However, its backbone is not made of sugars and phosphates like DNA’s but of a peptide-like chain. This makes PNA chemically resilient, especially in conditions where DNA would unravel and degrade.
This resilience is what caught the attention of Dr. Petkowski’s team. If any molecule could retain its integrity in the sulfuric acid-laden clouds of Venus, it might be PNA. So the researchers subjected PNA to a merciless test: a 98% sulfuric acid solution at room temperature, left to react for two weeks.
The results were startling. Not only did the PNA remain chemically intact—it retained its ability to interact with nucleic acids. This suggests that even in the harshest chemical conditions, complex informational molecules could persist, and potentially participate in the kind of biochemistry that underpins life.
Sulfuric Acid as a Solvent for Life
This finding builds upon a growing body of evidence that Venus’s atmosphere, despite being toxic and corrosive to life as we know it, could host an alternative chemistry. Previously, scientists had considered sulfuric acid to be purely destructive to organic molecules. But Petkowski and his colleagues have been steadily overturning that view.
“People think concentrated sulfuric acid destroys all organic molecules and therefore kills all life,” Dr. Petkowski said. “But this is not true. While many biochemicals, like sugars, are unstable in such an environment, our research shows that other chemicals found in living organisms, such as nitrogenous bases, amino acids, and some dipeptides, don’t break down.”
This changes everything. If molecules crucial to life—such as nucleic acids and amino acids—can survive in concentrated sulfuric acid, then perhaps that acidic medium isn’t the death knell we once assumed. Perhaps, under the right conditions, it could serve as a solvent for life, much as water does on Earth.
Echoes of Earlier Discoveries: Phosphine and Ammonia
The new study doesn’t arise in isolation. In 2020, a flurry of excitement surrounded the detection of phosphine gas in Venus’s atmosphere by researchers at Imperial College London. Phosphine is considered a potential biosignature—on Earth, it’s produced primarily by microbial life in oxygen-poor environments. Later that year, scientists from Cardiff University, including Dr. William Bains, detected ammonia, another potential indicator of biological processes.
Together, phosphine and ammonia painted a tantalizing portrait of Venus’s clouds—not as sterile and dead, but chemically active and potentially habitable. However, the idea met with skepticism. Critics pointed out that these gases could have non-biological origins and, more importantly, that Venus’s atmosphere seemed too harsh to support any complex organic molecules.
The new findings on PNA offer a powerful rebuttal to that skepticism. As Dr. Bains notes, “Both ammonia and phosphine are biomarkers… But Venus’s clouds are utterly hostile to life as we know it on Earth. So our latest study seeks to explore the potential of concentrated sulfuric acid as a solvent that could support the complex chemistry needed for life.”
The Beginning of a New Biochemistry
The implications of this research stretch far beyond Venus. It pushes scientists to think beyond Earth-centric models of life and to consider the possibility that life elsewhere might be built on an entirely different scaffold. If a molecule like PNA can survive—and even thrive—in a medium as corrosive as sulfuric acid, it may serve as a template for exploring life in other harsh environments, from the acidic oceans of Europa to the hydrocarbon lakes of Titan.
Dr. Petkowski sees this as the dawn of a new scientific domain. “We’ve started a new chapter on the potential of sulfuric acid as a solvent for life,” he says. “This is just the beginning.”
But the study also acknowledges its limitations. While PNA can survive in concentrated sulfuric acid at room temperature, it breaks down at higher temperatures—above 50°C. Given that the upper layers of Venus’s cloud deck span a temperature range between 0°C and 100°C, this poses a significant challenge. Future research, the team says, will focus on designing or identifying a genetic polymer—a molecule capable of encoding information—that is stable throughout this entire temperature range.
Engineering Molecules for Alien Environments
This line of research represents a shift in astrobiology: not just looking for molecules that survive alien environments, but engineering them to do so. The field is beginning to explore the idea of life as a universal phenomenon that might arise under radically different chemical conditions—what some scientists call “weird life.”
To do that, researchers will have to break free from the biological dogmas imposed by life on Earth. DNA and water may be the scaffolding of life here, but elsewhere, life may be built on PNA and sulfuric acid. Such possibilities have long been the realm of science fiction, but studies like this one are bringing them into the domain of experimental science.
Industrial and Scientific Spin-Offs
The discoveries also hold promise for industries far removed from astrobiology. Sulfuric acid is one of the most commonly used industrial chemicals on Earth, central to processes ranging from fertilizer production to metal refining. Understanding how complex molecules behave in this medium could lead to innovations in materials science, chemical synthesis, and biotechnology.
“This research forces us to rethink the chemistry of sulfuric acid,” said one team member. “If we can learn how to design stable, information-carrying molecules that function in this environment, we may open the door to whole new classes of chemical tools for use in extreme conditions.”
The Bigger Picture: Life, the Universe, and Everything
For now, the clouds of Venus remain tantalizing and mysterious. No mission has yet returned samples from the planet’s atmosphere, though several space agencies—including NASA, ESA, and India’s ISRO—are developing Venus probes that may one day bring us closer to the truth. These missions could finally test whether molecules like PNA—or even life itself—float within Venus’s toxic clouds.
The vision of life suspended in acid, clinging to chemically engineered molecules, may seem far-fetched. But so too did the idea of extremophiles on Earth—organisms that thrive in boiling sulfur springs, beneath crushing oceanic pressure, or in radioactive waste. Nature, as science keeps reminding us, is full of surprises.
And perhaps Venus is preparing to surprise us once again.
Conclusion: A Universe Full of Possibilities
The notion that the clouds of Venus—a place once dismissed as a chemical death zone—might harbor the building blocks of alien life is nothing short of revolutionary. This new study, demonstrating the resilience of PNA in sulfuric acid, hints at an astonishing possibility: that life may not be unique to Earth, nor even to water-rich environments.
Instead, life may be a tenacious force, capable of adapting to even the most hostile corners of the universe—provided it has the right molecular tools.
As we refine our understanding of biochemistry, redefine the meaning of habitability, and reimagine life itself, Venus beckons us with a powerful lesson: to look beyond the familiar, to embrace the strange, and to never stop asking what might be possible in the vast, alien reaches of the cosmos.
Reference: Janusz J. Petkowski et al, Astrobiological implications of the stability and reactivity of peptide nucleic acid (PNA) in concentrated sulfuric acid, Science Advances (2025). DOI: 10.1126/sciadv.adr0006